Entamoeba histolytica is a human intestinal protozoa which occurs throughout the world. Interestingly, only 10% of the infections causes diseases such as dysentery or liver abscess whereas the rest do not develop any clinical symptom. This uncorrelation suggested the presence of at least two distinct Entamoeba with indistinguishable morphologies, but with different pathogenicities. Thus, extensive researches have been performed to distinguish the nonpathogenic Entamoeba from pathogenic one using diverse laboratory methods (Sargeaunt et al., 1978; Tachibana et al., 1991a). Recently, nonpathogenic Entamoeba was differentiated from pathogenic E. histolytica and designated newly as Entamoeba dispar (Sargeaunt, 1992; Diamond and Clark, 1993).
In 1995, we reported the axenization of YS-27, a Korean Entamoeba strain (Chang et al., 1995). Isolated from a patient with a hepatic abscess in 1969, this strain had been maintained as a xenic culture for more than 15 years. Through monoxenic cultivation with Crithidia, the axenization of YS-27 was accomplished in TYI-S-33 medium (Diamond et al., 1978). Here, we describe further characterization of the YS-27 strain using three different laboratory methods.
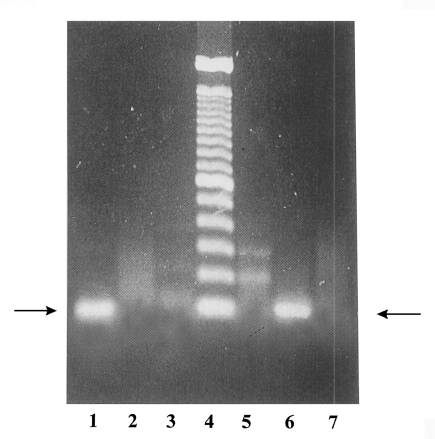
Polymerase chain reaction (PCR) was employed to characterize the YS-27 by using four primers, p11, p12, p13, and p14, designed by Tachibana et al. (1991b). Two primers, p11 and p12, were based on the 30 kDa molecule, which is present only in E. histolytica. The others, p13 and p14, were employed to detect E. dispar (Tachibana et al., 1990, 1991a). YS-17 strain which had been isolated from a cyst-carrier of Severance Hospital in 1968 was used as a positive control for E. dispar.
As shown in Fig. 1, PCR of YS-27 DNA with p11 and p12 showed the 100 bp DNA band corresponding to the partial gene for the 30 kDa molecules. On the other hand, PCR product of YS-17 DNA did not show that band. When p13 and p14 were used as primers, YS-27 DNA did not show any band whereas YS-17 DNA showed a specific band. This result indicated that YS-27 is E. histolytica and YS-17 is E. dispar. PCR-RFLP study using the P1 gene-specific primers drawed the same conclusion about YS-27 (Choe et al., 1996).
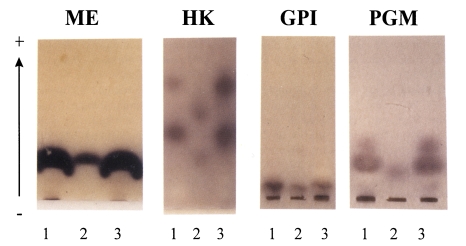
As an alternative tool to differentiate the pathogenic Entamoeba from nonpathogenic one, the mobility patterns of some metabolic enzymes on starch gel have been employed. More than twenty distinct groups of Entamoeba were demonstrated using four enzymes, hexokinase (HK), glucose phosphate isomerase (GPI), phosphoglucomutase (PGM), and L-malate:NADP oxidoreductase (ME) (Sargeaunt, 1985).
Lysates of YS-27 trophozoites were prepared as previously described (Matias et al., 1991). The soluble protein extracts were subjected to horizontal starch gel electrophoresis by the method of Wraxall and Culliford (1968). Then, the gels were exposed to the reaction solutions containing the substrates, cofactors, and visualization reagents. According to the enzymes interested, the reaction solutions were varied as described (Sargeaunt, 1985).
While one band of ME with a same mobility was found in both Entamoeba, YS-27 and YS-17, different isozyme patterns were observed for HK between these two strains (Fig. 2). In YS-27, two HK enzymes moved faster than those in YS-17. In cases of GPI, lysates of YS-27 or YS-17 showed a single band with a slow mobility (Fig. 2). For PGM, two bands were observed in YS-27 lysate whereas a slow-moving band was found in YS-17. The isozyme patterns of YS-27 are exactly as same as those of group II E. histolytica HM1:IMSS in all of the four enzymes. Thus, we could conclude that YS-27 strain belongs to E. histolytica group II like HM1:IMSS strain. On the other hand, YS-17 strain was found to be group I E. dispar.
To examine the pathogenicity of YS-27, which has been subcultured in vitro for about 30 years, we used the Mongolian gerbil model for the study of amebic liver abscess formation. Saline-washed trophozoites (1×106) were injected intraperitoneally to three 3 day-old Mongolian gerbils (Meriones unguiculatus). At 3 days postinoculation, the gerbils were sacrificed, and the livers of the animals were observed for the presence of abscesses. Two to four small liver abscesses (2 mm in diameter) were found from all the three gerbils. The removed abscesses were put in 0.85% saline and examined under a light microscope. Motile trophozoites of amoeba were observed from the abscess fluid.
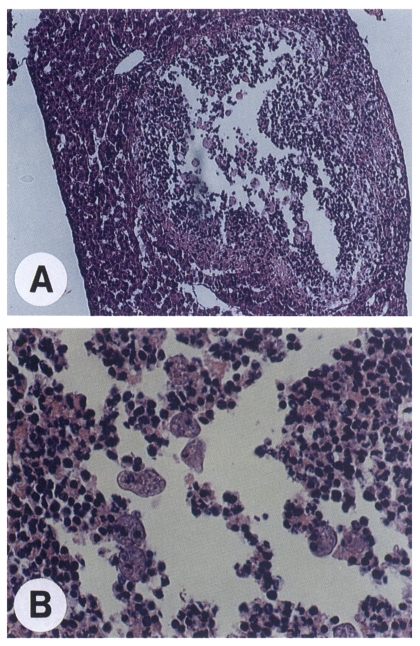
Haematoxylin and eosin staining of the infected liver tissues allowed us histological assessment of the amoeba-invaded area as well as confirmation of the presence of the amoeba. Abscesses were characterized by a central lytic necrosis containing scattered amoebae (Fig. 3). This necrotic region was surrounded by inflammatory cells including neutrophils which formed a boundary to separate the necrotic area from the normal liver parenchyma.
The present results reconfirmed that YS-27 is E. hisolytica. PCR with species-specific primers and zymodeme analysis indicated that YS-27 belongs to a pathogenic zymodeme group II. Moreover, the pathogenicity of YS-27 strain was directly shown in the experimental infection study.