Spirometra erinacei is an intestinal cestode parasite found in cats and dogs. Its life cycle stages include eggs, coracidium, procercoid in crustaceans, plerocercoid (sparganum) in terrestrial vertebrates and adults in carnivorous mammals (Lee et al., 1990; Cho, 1996). This cestode is regarded as one of important human-infecting parasites because human sparganosis occurs worldwide especially in East and Southeast Asian countries. Once infected, sparganum migrates through the tissues, forming a tortuous track and granuloma around it; sometimes the larva invades human brain and causes several neurological symptoms (Chang et al., 1992).
Migrating ability of sparganum through the host tissue has long been considered to be associated with secreted proteolytic enzymes. Presently, three species of serine proteases (Kong et al., 1994a) and three species of cysteine proteases have been elucidated in the sparganum (Fukase et al., 1985; Song et al. 1993; Kong et al., 1997). Of these, 27 kDa cathepsin L-like enzyme has been found to be most important in tissue invasion and nutrient uptake and its biochemical and structural nature has well been characterized (Kong et al., 1994b; Liu et al., 1996). The cysteine proteases also modulate host immune response by cleaving immunoglobulins or by provoking IgE antibody responses. In this study, we demonstrated that expression of the gene encoding 27 kDa cathepsin L-like cysteine protease is stage-specifically regulated.
Sparganum was harvested from naturally infected snake, Elaphe rufodorsata and used to experimentally infect the dog. Adult S. erinacei were collected from the dog which was orally infected with two sparganum and was allowed to grow for two months. Two weeks after the experimental infection, immature eggs of S. erinacei were collected from dog stool. The purified immature eggs were used for either the protein extraction or coracidial hatching. The coracidium was obtained by hatching the eggs in a 29℃ incubator (Lee et al., 1990). Procercoid larva derived from coracidium-infected freshwater copepodes, Mesocyclops leuckarti and Eucyclops serrulatus were used to infect tadpoles and rats. Plerocercoids were harvested from either hosts at three weeks after infection. All stages of S. erinacei were stored in liquid nitrogen or in -70℃ deep freezer until RNA preparation or protein extraction.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed to compare the mRNA expression level of the 27 kDa cysteine protease gene. Total RNA samples isolated from immature egg, coracidium, sparganum and adult were quantified by spectrophotometer at 260 nm and by DNA Dipstick (Invitrogen, Carlsbad, CA, USA). Two microgram total RNA of each sample was reverse transcribed using oligo d(T)15 primer and Molony murine leukemia virus (M-MuLV) reverse transcriptase (Life Technologies, Gaithersburg, USA). RT-PCR was carried out at 42℃ for 90 min. Aliquots of the resulting cDNA were subjected to PCR amplification with gene specific primers encoding the cathepsin L-like cysteine protease of sparganum; sense, 5'-CTGAAAGTGAGACGTACGTC-3' (SeCp70) and antisense, 5'-CAGCTGCAGTCCATCAACTG-3' (SeCp541) (D63670, Liu et al., 1996). The cycling parameters for PCR were 94℃ for 2 min, followed by 35 cycles of 94℃ for 45 sec, 58℃ for 45 sec and 72.5℃ for 1 min with a final incubation at 72.5℃ for 10 min. The PCR condition was kept identical in all reaction. The PCR products were analysed on 1.3% agarose gel. The test was repeated three times to confirm the expression of the cDNA. Northern blot analysis was also performed with the use of 32P-labeled full-length cDNA. The full-length cDNA was obtained by PCR cloning. Ten microgram each of total RNAs of sparganum (each from frog and rat) and adult were separated on 1.2% formaldehyde agarose gel and transferred to Hybond N+ membrane (Amersham-Pharmacia Biotech, Sweden) by capillary action. Labeling of the probe and detection of hybridization signal were carried out using 32P-labeled Rediprime DNA Labelling kit under conditions recommended by the manufacturer (Amersham-Pharmacia Biotech). Hybridization was done with 50% formamide/6× SSC solution for 16 hr at 50℃. After washing with high stringency, the membrane was autoradiographed (Sambrook et al., 1989).
The proteolytic activities of cysteine protease in the crude extracts of immature egg, coracidium, sparganum and adult were assayed fluorometrically using synthetic substrate, carboxybenzoyl-phenylalanyl-arginyl-7-amino-4-methylcoumarin (Cbz-phe-arg- AMC, in 0.1 M sodium phosphate buffer, pH 5.7), in the presence of 5 mM dithiothreitol (DTT, Sigma, St. Louis, MO, USA). Excitation and emission wave-length was 380 nm and 460 nm, respectively. One unit of specific activity was defined as the amount of enzyme that release 1 micromole AMC per 1 hr (Chung et al., 1997).
Modulation of the enzyme activity was observed using inhibitors acting on cysteine protease, transepoxy-succinyl-L-leucylamido(4-guanidino) butane (E-64, 10-5 M) and iodoacetic acid (IAA, 1 mM), serine protease, aprotinin (10 µM), diisopropyl fluorophosphate (DFP, 1 mM), soybean trypsin inhibitor (SBTI, 1 mM), phenylmethyl sulfonyl fluoride (PMSF, 1 mM), N-α-p-tosyl-L-lysine chloromethylketone (TLCK, 1 mM) and L-1-tosyl-amido-2-phenyl(ethyl) chloromethylketone (TPCK, 1 mM) and metalloprotease, ethylenediaminetetraacetic acid (EDTA, 2 mM) and 1,10-phenanthroline (2 mM). All the chemicals used were from Sigma except for E-64 which was from Enzyme System Products (Livermore, CA, USA). Prior to standard assay of enzyme activity, respective inhibitors were incubated with the enzyme solution for 30 min at a room temperature (Kong et al., 1994b).
As shown in Table 1, the extracts from coracidia and spargana exhibited cysteine protease activity while those of immature eggs and adult worms did not. The activity appeared to be the highest in the extracts prepared from scolices of spargana, but the enzyme activity in coracidial extracts was about one fifth of that in rat sparganum. Table 2 shows the modulation of enzyme activity by inhibitors acting on the coracidial extracts. The activity was inhibited specifically by cysteine protease inhibitors such as E-64 and IAA at the concentrations commonly used in the assays. Meanwhile, inhibitors acting on serine- and metallo-proteases such as aprotinin, DFP, SBTI, PMSF, TLCK, TPCK, EDTA and 1,10-phenanthroline did not affect the enzyme activity. Although we were not able purify the cysteine protease from the coracidia due to insufficient amount of protein available (about 250 µg), the results clearly showed the presence of cysteine protease activity in the coracidial extracts particularly, and this was confirmed with a molecular substrate that shows specificity towards cysteine protease, Cbz-Phe-Arg-AMC (Barrett & Kirshke, 1981).
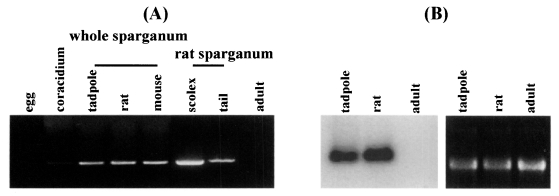
Semi-quantitative RT-PCR was carried out with the 27 kDa cysteine protease gene specific primers to examine the expression levels in immature eggs, coracidium, sparganum and adult. Expression of the 27 kDa cysteine protease was detected in the extracts of coracidia and spargana but not in those of immature eggs and adult. The RNA extracted from sparganum scolex showed higher expression level than that from tails (Fig. 1A). Northern hybridization demonstrated that the RNA isolated from sparganum obtained from rat showed higher levels of expression than that from tadpoles. The RNA extracted from the adult worms exhibited no signals (Fig. 1B). Despite a few inconsistencies due to varying sensitivities of the tests employed in this study, the results coincided well with the data obtained by protease assay as seen in Table 1.
It has been speculated that the sparganum may express protective/defensive proteins due to its low host-parasite specificity (Cho, 1996). One of the plausible mechanisms for the successful adaptation of the sparganum is its ability to express certain proteolytic enzymes. In this study, we have demonstrated that the 27 kDa cysteine protease of S. erinacei was expressed specifically in the embryo (coracidium) and plerocercoid stages. The present result indicated that the 27 kDa cysteine protease is expressed only when penetration and active migration in the host tissues are necessary. The coracidium must penetrate the copepod gut wall to develop into the procercoid, and the plerocercoid must penetrate the gut wall of various species of vertebrate hosts to be transferred to another transport hosts. The roles and developmental modulation of cysteine proteases have been known in many helminth parasites including Paragonimus westermani and Haemonchus contortus (Chung et al., 1997; Skuce et al., 1999).
The sparganum has been shown to express cysteine proteases of different species such as 53 kDa and 21 kDa in addition to 27 kDa (Fukase et al., 1985; Kong et al., 1997). Further studies will be necessary to elucidate the different biological reactivities of the respective enzymes other than 27 kDa protease during the process of parasite maturation and adaptation in the various hosts.