INTRODUCTION
Hydatidosis is a chronic infection of medical and veterinary importance caused by the larval stage of a cosmopolitan parasitic cestode Echinococcus granulosus [1,2]. Cystic hydatid disease(CHD) is of public health importance not only in areas of endemicity but also in countries without endemicity due to migration of infected people and livestock exchanges which create new areas of endemicity [3]. Unfortunately, numerous reports indicate that hydatidosis incidence has increased in various regions of the world [4]. In Egypt, infection with the E. granulosus larval stage is a serious zoonotic helminthic disease [5]. This is why Egypt started to be considered as an emerging endemic area of cystic echinococcosis [6].
Hydatid cyst is distributed throughout most of the world, especially in areas where sheep are raised, and is endemic in Asia, North Africa, South and Central Americas, North America, Canada, and the Mediterranean region. In many countries, hydatid disease is more prevalent in rural areas where there is close contact between people, dogs, and various domestic animals which act as intermediate hosts [7].
Since the mid-1970s, significant advances have been made in the chemotherapy of the metacestode stage of Echinococcus. In vitro and in vivo techniques for drug testing are now available, and considerable clinical, pharmacological, and parasitological knowledge has accumulated [8]. Benzimidazole carbamate derivatives, such as albendazole and mebendazole, in addition to amphotericin B and praziquantel, are currently the drugs of choice for treatment of cystic echinococcosis [9]. Nevertheless, the recurrence rates after interruption of therapy are high and new options for chemotherapeutic treatment are needed [10].
Deger et al. [11] demonstrated that albendazole sulfoxide injection, as a scolicidal agent in the percutaneous treatment of cystic echinococcosis, seems to be effective in sheep. Treatment and control of parasitic diseases, including helminthiases, are usually made by synthetic anthelmintics. The appearance of resistance to synthetic anthelmintics stimulated the research for alternatives, such as medicinal plants [12]. According to circumstances and depending on their efficacy, naturally produced plant anthelmintics offer an alternative that can overcome some of these problems and is both sustainable and environmentally acceptable [13].
Thyme, mentha, and salvia are very popular medicinal plants in the Middle East as well as all over the world with many folkloristic uses. Their active constituents may be responsible for their pharmacological effects [14]. Thyme and salvia are 2 famous Egyptian plants containing essential oils, while thymol and menthol are 2 major isolates of these volatile oils; these were found to inhibit the growth of a wide range of organisms that cause various diseases [15,16]. Recent studies have shown that sage and its constituents have antibacterial actions against various microorganisms [17]. Thyme is used as an antimicrobial [18,19], anti-amoebic [20], and anti-inflammatory drug [21].
As the Egyptian flora is rich in plants, the possibility of finding new antimicrobial agents is still widely ahead, therefore, the aim of the present study was to determine in vitro protoscolicidal effects of different concentrations of alcoholic extracts of salvia and thyme, in addition to thymol and menthol pure compounds, on the viability of protoscolices of E. granulosus in comparison with control groups.
MATERIALS AND METHODS
Parasite material
Hydatid cysts of E. granulosus were removed from lungs and livers of naturally infected camels slaughtered in Bani-Adi and El-Atamina abattoirs, Assiut Governorate, Egypt. The intact cysts were placed in an ice box, and transported within 3 hr to Parasitology laboratory, Department of Parasitology, Faculty of Medicine, Assiut University, Assiut, Egypt.
Collection of hydatid fluid
Cysts were washed several times in sterile PBS, pH 7.2. Cyst surfaces were sterilized by 70% ethyl alcohol and their fertility was determined by the presence of free protoscolices in cystic fluid by microscopic examination of a wet mount drop. Hydatid fluid with protoscolices was collected as previously described by Smyth [22].
Preparation of protoscolices
Hydatid fluid containing protoscolices was evacuated completely into 15 ml Falcon tubes without centrifugation and it was left to precipitate for an hour to obtain hydatid sand at room temperature. Protoscolices were maintained in a sterile preservative solution made of a mixture of Krebs-Ringer solution (KRS) and hydatid cyst fluid (4:1) [23] for all experiments. This preservative solution dose not contain any antibiotic or antifungal drugs.
The viability of protoscolices was determined prior to the experiments. A 0.01 ml solution of pooled protoscolices was transferred over a slide and mixed with 0.01 ml of 0.1% aqueous eosin stain, as a vital staining, and was evaluated by low power microscopy after 5 min. Unstained protoscolices were considered as viable while stained protoscolices were considered as non-viable [24]. When the percentage of viable protoscolices in the sediment was 95%or more, they were considered to be appropriate for experiments. The percentage of viable protoscolices (viability rate) was determined by counting a minimum of 100 protoscolices (as a ratio of number of viable protoscolices to total protoscolices).
Plant collection and extraction
The aerial parts of salvia (Salvia officinalis), also known as saline, purple, or sage, and thyme (Thymus vulgaris) (750 mg, each) were collected from a local market in Assiut Governorate, Assiut, Egypt in March 2010. The plants were identified by Department of Pharmacognosy, Faculty of Pharmacy, Assiut University, Assiut, Egypt. A voucher specimens were deposited in the Botanical Museum, Faculty of Pharmacy, Assiut University, Assuit, Egypt.
A 500 g of the shade-dried powdered aerial parts of both plants were extracted separately with 70% ethanol by maceration and percolation for 24 hr. The process of extractions was repeated twice. The alcohol extract of each plant was pooled together and evaporated under reduced pressure at 45℃ till free from solvent. The alcohol free residue of each extract was weighted to give 9.66 g in case of salvia and 8.54 g in case of thyme. Preliminary phytochemical tests were carried out to identify the main constituents of each extract [25-27]. Thymol was purchased from BDH Chemicals LTD (Poole, UK). Menthol was purchased from El-Nasr Company for Pharmaceuticals and Chemicals (Cairo, Egypt).
Determination of in vitro effects
Stock solutions of alcoholic crude extracts of salvia, thyme, thymol, and menthol were prepared by dissolving 2.5 g of the dried extract or pure compound in 10 ml distilled water (25 mg/ml). Adding a few drops to 5 ml Tween 80 (2%) helped dissolving the dried extract or pure compound. Concentrations of 500, 1,000, 1,500, and 2,500 µg/ml of stock solutions for salvia (experiment 1) and thyme (experiment 2) were used. Concentrations of 1, 10, and 50 µg/ml of the stock solutions for thymol (experiment 3) and menthol (experiment 4) were used. The efficacy of alcoholic extracts of both plants on the viability of protoscolices was compared with that of albendazole sulfoxide (ABZSO) 800 µg/ml (experiment 5), the recommended drug for treating hydatidosis [28]. Sterile preservative solution and Tween 80 (2%) were used as negative controls.
The in vitro study was carried out in sets of 26 Falcon tubes. A 5.0-ml of the preservative solution was added to each tube. A 1.0-ml solution of viable protoscolices (approximately 2,000 viable protoscolices) was placed in each tube, and 1.0 ml of each experimental preparation (experiments 1-5) was added to each tube, mixed gently, and incubated at 30℃ without changes of preservative solution. Percentages of viable protoscolices or survival were determined for each experiment after 5 min and in serial period of time (every 12 hr) until all of the protoscolices were dead. The data were derived from the mean value of 3 replications.
RESULTS
Viability of protoscolices
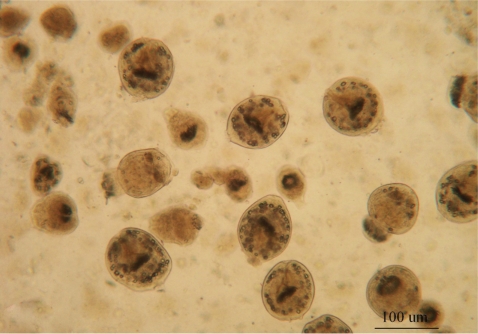
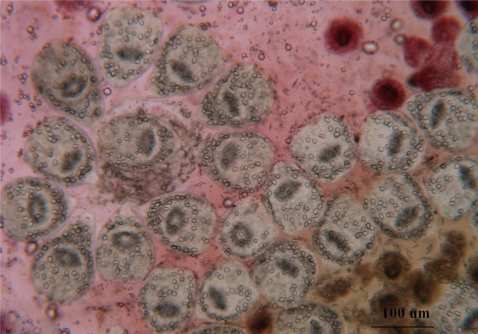
The fertility (=reproducibility) of hydatid cysts was determined by the presence of free protoscolices in the cystic fluid by wet mount drop (Fig. 1). The viability of protoscolices was tested prior to the experiments using 0.1% aqueous eosin stain. Dead protoscolices absorbed eosin and colored red, but alive protoscolices remained colorless and showed characteristic muscular movements and flame cell activity (evaluated under light microscope) (Fig. 2).
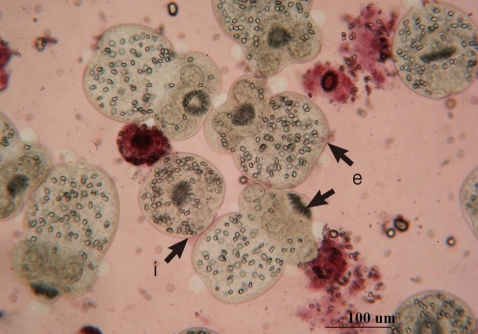
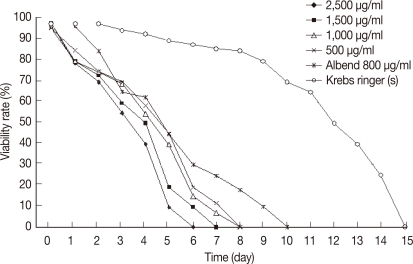
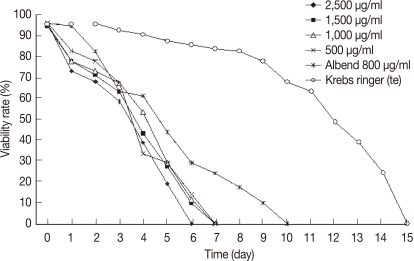
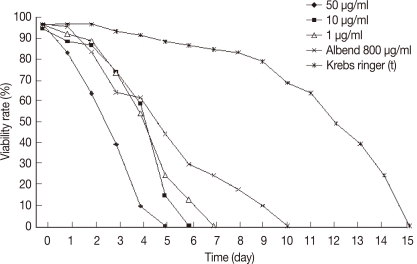
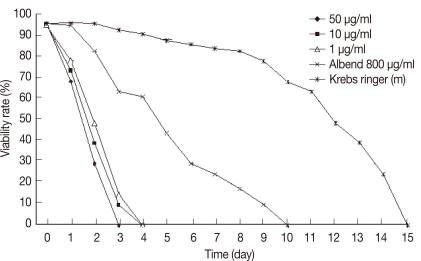
Protoscolices remained viable for average 15 days after being incubated in sterile preservative solution (negative control) at 30℃, without treatment, with minimal differences in viability between the first and 10th day (P<0.05), while starting from the 11th day, there was significant decrease in the viability of protoscolices till total death of all protoscolices occurred by the end of the 15th day (Fig. 7). In the sterile preservative solution, microscopic examination of viable protoscolices exhibited distinct movements and conserved with the membrane integrity and order of hooks. Most of these protoscolices were changed into an evaginated form (scolices) by time, and suckers were clearly visible (Fig. 3).
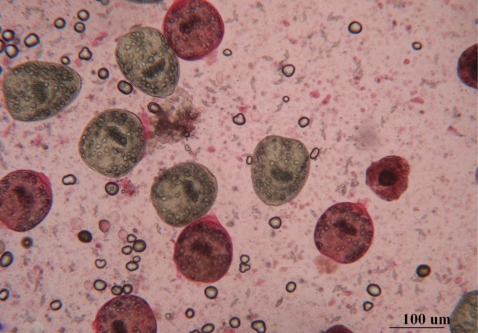
Microscopic examinations of dead protoscolices showed distortion of their morphology and degenerative effects. These effects were characterized by loss of motility, loss of hooks, or presence of free hooks and calcareous corpuscles (Fig. 4). The viability of protoscolices incubated with 2% Tween 80 showed the same effects as that incubated in the preservative solution.
In vitro treatment of protoscolices
The viability of protoscolices was significantly affected in experiment 1 (salvia). The protoscolices treated with concentrations of 2,500, 1,500, 1,000, or 500 µg/ml started to show a decrease in viability at day 1 post-treatment (PT) (Fig. 5). All treated protoscolices died between days 6 and 8 PT (Fig. 7). The highest concentration (2,500 µg/ml) showed the best result where all protoscolices died at day 6 PT. This result was significant(P<0.05). In experiment 2 (thyme): all protoscolices died at day 6 PT when treated with 2,500 µg/ml, while protoscolices died at day 7 PT when treated with the other concentrations (1,500, 1,000, or 500 µg/ml). These results were also significantly different between groups (P<0.05) (Fig. 8). Both alcoholic extracts of salvia and thyme inhibited any evagination of the protoscolices in addition to dose-dependent scolicidal activity (Fig. 6).
In experiment 3 (thymol): all protoscolices treated at concentrations 10 or 1 µg/ml, died at days 6 and 7 PT, respectively. While, protoscolices treated with thymol at the concentration of 50 µg/ml died at day 5 PT (P<0.05) (Fig. 9). In experiment 4 (menthol): all protoscolices treated at concentrations of 10 or 1 µg/ml died at day 4 PT, while protoscolices treated with menthol at the concentration of 50 µg/ml died at day 3 PT (P<0.05) (Fig. 10).
In experiment 5 (albendazole): a significant effect on the viability of protoscolices was exhibited at day 6 from the onset of treatment, where 70% of the incubated protoscolices died. All of the remaining protoscolices died by the end of day 10 PT. All protoscolices remained invaginated in albendazole-treated groups. Negative controls also showed a decline in viability; however, 80.2% of the protoscolices were still viable by day 10 PT. A comparison between all experimental results is summarized in Table 1. The preliminary phytochemical screening of both plant extracts showed the presence of volatile constituents, sterols, and/or terpenes, tannins, flavonoids, lactones, and/or esters, coumarins, and saponins using standard procedures.
DISCUSSION
Despite some progress in the control of echinococcosis, this cestode zoonosis continues to be a major public health problem in several countries, and in several others, it constitutes an emerging and re-emerging disease [29]. It has been recognized that hydatid protoscolices remain viable for a long period in different media at different temperatures. In the absence of nutritive factors, the protoscolices of E. granulosus do not survive in vitro for more than 4 days at 37℃. This survival time depends on temperature, pH, and ionic concentration [30]. In the present study, hydatid protoscolices remained viable for average 15 days after being incubated in sterile preservative solution at 30℃.
The results of our study agreed to the previous in vitro studies [23,31] which reported that Krebs-Ringer solution with hydatid cystic fluid 4:1 was the best preservative medium for protoscolices. This may be due to the fact that Krebs-Ringer solution has nutritional factors and a number of minerals (glucose, magnesium chloride, potassium chloride, sodium chloride, sodium phosphate dibasic, and sodium phosphate monobasic) that provide protoscolices with the minimal requirements to keep them alive for a long period of time [32]. Another culture media (M-199), supplemented with 100 IU penicillin, 100 µg/ml streptomycin, and 4 mg/ml glucose, has been reported to keep protoscolices alive more than 86 days [13]. This may be explained by the presence of antibiotics, whereas Krebs-Ringer solution dose not contain any antibiotic or antifungal drugs.
In addition to survival of protoscolices in the preservative solution, there was evagination of some protoscolices. This could be explained as a step starting their development in the preservative solution. Alsudani [30] reported similar morphological changes in culture media. The viability assessment in cystic echinococcosis is important for in vitro and in vivo studies. Several vital and avital stains, like neutral red, methylene blue, eosin, Giemsa, Ziehl-Neelsen, and trypan blue, have been used to assess viability of protoscolices in fertile cysts [33]. In the present study, 0.1% aqueous eosin stain was used in viability assessment of hydatid cysts and in assessment of the effects of used drugs on viability of protoscolices in vitro. The viable protoscolices were colourless and dead ones absorbed the red colour of aqueous eosin.
Cystic echinococcosis is among the most neglected parasitic diseases, and development of new drugs and other treatment modalities received very little attention. Clinical management procedures had evolved over decades without adequate evaluation of important features, such as efficacy, effectiveness, rate of adverse reactions, relapse rate, and cost [34]. To date, many scolicidal agents have been used for inactivation of the hydatid cyst content, but there has been no ideal agent that is both effective and safe [1]. The use of plant extracts against protoscolices of hydatid cyst has received critical attention in recent years. Some studies have shown that extracts of certain plant species belonging to different families may affect the viability of protoscolices and/or survival of the secondary hydatid cyst [35].
The antimicrobial properties of plant extracts have been well established against wide spectra of microbes, including bacteria and fungi, using a variety of experimental methods. Most of the antimicrobial activity in medicinal plants appear to be derived from phenolic compounds [8]. The present results showed that the alcoholic extract of both salvia and thyme are effective on the viability of protoscolices. The highest concentration (2,500 µg/ml) of both extracts showed the best results, where all protoscolices died by day 6 PT. Other concentrations (1,500, 1,000, and 500 µg/ml) of salvia and thyme extracts caused death of protoscolices also but needed a longer period than the highest concentration. The dose-dependent protoscolicidal effects of these medicinal plant extracts seem to be due to their role in breakdown of biological activities of protoscolices through interference with their metabolism. They may have target sites, such as inhibitors of protein or DNA synthesis, or within cytoplasmic components, like B-lactam antibiotics [36,37].
Several essential oils and their constituents have been found to possess anthelmintic activities [38,39]. Essential oils are natural products extracted from vegetal materials, which have antibacterial, antifungal, antioxidant [40,41], or antiparasitic properties [42]. The present study showed that all protoscolices treated with thymol at concentration of 10 µg/ml and 1 µg/ml died at days 6 and 7 PT, respectively, while protoscolices treated with menthol at the same concentration died at day 4 PT. On the other hand, protoscolices treated with 50 µg/ml of both thymol and menthol died at days 5 and 3 PT, respectively, which means that menthol at 50 µg/ml had the best effect on the viability of protoscolices followed by thymol at the same concentration. These results agree with the report of Elissondo et al. [13] who studied the efficacy of thymol against E. granulosus protoscolices and reported that the maximum protoscolicidal effect was found with 10 µg/ml concentration. Elissondo et al. [13] used protoscolices of cattle strain obtained from an abattoir located in the southeast of the Buenos Aires Province, Argentina. In our study, we used protoscolices of camel strain obtained from Bani-Adi and El-Atamina abattoirs, Assiut Governorate, Egypt. The difference in the strain of the protoscolices of E. granulosus and in the locality (more fragile strain) could be responsible for this discrepancy.
Thymol is one of the major components of essential oils of thyme and is a widely known anti-microbial agent. It has also been tested as a scolicidal agent in some studies and showed an excellent effect [13]. It has the ability to partition in the membrane from an aqueous phase as well as a capacity to affect the membrane organization and the surface electrostatics [43]. The ideal treatment for patients with hydatid disease should be complete elimination of the parasite and prevent recurrence of the disease with minimal morbidity and mortality. Available therapeutic modalities for hydatid cysts consist of systemic chemotherapy, surgery, and percutaneous treatment [44]. Other plant extracts tested for scolicidal effects include garlic Allium sativum [45], Pistacia khinju, Zizyphus spina-Christ, Olea earpaea [30], Zataria multiflora [8], Dendrosicyos socotrana, Jatropha unicostata [35], and Propolis [46]. They showed potent scolicidal effects.
Results during the present work pointed out that the medicinal plant extracts of thyme and salvia, in addition to pure compounds, are potent scolicidal agents at different concentrations. The dose-dependent efficacy of these plant extracts and pure compounds appeared to be more effective than that of albendazole which did not induce its scolicidal effect before day 10 PT.
Our data suggest that medicinal plants can be a promising source of potent antiprotoscolices. We recommend future works on the extracts and other pure compounds from these 2 plants to find another antiparasitic agents that are not affected when treated with common therapeutic agents.