AbstractWe have reported that a 24 kDa protein (22U homologous; As22U) of Anisakis simplex larvae could elicit several Th2-related chemokine gene expressions in the intestinal epithelial cell line which means that As22U may play a role as an allergen. In order to determine the contribution of As22U to allergic reactions, we treated mice with 6 times intra-nasal application of recombinant As22U (rAs22U). In the group challenged with rAs22U and ovalbumin (OVA), the number of eosinophils in the bronchial alveolar lavage fluid (BALF) was significantly increased, as compared to the group receiving only OVA. In addition, mice treated with rAs22U and OVA showed significantly increased airway hyperresponsiveness. Thus, severe inflammation around the airway and immune cell recruitment was observed in mice treated with rAs22U plus OVA. The levels of IL-4, IL-5, and IL-13 cytokines in the BALF increased significantly after treatment with rAs22U and OVA. Similarly, the levels of anti-OVA specific IgE and IgG1 increased in mice treated with rAs22U and OVA, compared to those treated only with OVA. The Gro-α (CXCL1) gene expression in mouse lung epithelial cells increased instantly after treatment with rAs22U, and allergy-specific chemokines eotaxin (CCL11) and thymus-and-activation-regulated-chemokine (CCL17) gene expressions significantly increased at 6 hr after treatment. In conclusion, rAs22U may induce airway allergic inflammation, as the result of enhanced Th2 and Th17 responses.
INTRODUCTION
Anisakis and Pseudoterranova are the 2 nematode genera that are most frequently associated with human anisakidosis. Any fish or cephalopod species can be parasitized by the 3rd stages of these larvae. The ingestion of the 3rd stage larvae can also induce anisakidosis in humans [1]. Symptoms of anisakidosis arise when the nematode penetrates the gastric mucosa, which results in acute epigastric pain, occasionally accompanied by nausea and vomiting. Another common manifestation of human anisakidosis is an IgE-mediated immune reaction that sometimes occurs in sensitized individuals. Anisakis has been implicated in a range of allergic diseases, including dermatitis, asthma, and food allergy [2-4]. It has been estimated that 7% to 36% of seafood processing workers develop occupational asthma, while 3% to 11% have urticaria and atopic or protein contact dermatitis [5]. In fact, as many as 15% of adult asthma cases are related to occupational exposure [2].
Live larvae can also cause gastrointestinal diseases in humans. However, whether direct exposure to Anisakis antigens can directly lead to systemic allergic sensitization is yet to be demonstrated [2]. Sensitization to Anisakis may occur via ingestion of infected fish, inhalation of airborne Anisakis allergens or direct contact with Anisakis proteins in fish [6]. Therefore, some allergens might directly lead to systemic allergic responses. As A. simplex allergens, the following 12 protein types have been identified to date; secretory gland protein (Ani s 1) [7], myosin (Ani s 2, 3) [8,9], protease inhibitors (Ani s 4, 6) [10,11], the SXP/RAL-2 family proteins (Ani s 5, 8, 9) [11-13], and proteins with repetitive sequences (Ani s 7,10-12) [14-16]. In addition to these identified allergens, there might be many other unknown allergens.
In a previous study, we identified the As22U protein from the 3rd stage larvae of Anisakis simplex [17]. The function of this protein is not exactly known, but it may influence the host, because it was found in the group of excretory-secretory (ES) proteins [17]. In addition, we found that they could elicit Th2-related chemokine gene expression in the intestinal epithelial cells. However, we did not evaluate its allergenic activity in vivo animal model. Experimental respiratory allergens are distinguished by their ability to elicit allergic lung inflammation when inhaled. Ovalbumin (OVA) is a commonly used experimental allergen, incapable of eliciting allergic inflammations if administered strictly by means of inhalation, whereas pollen and fungal-derived allergens readily induce allergic responses when administered through the respiratory tract [18-20]. Therefore, if As22U has allergen properties, repeated administration through the respiratory tract could elicit allergic airway inflammation.
In this study, in order to investigate whether As22U has allergic properties, we constructed recombinant As22U (rAs22U) and administrated it to the mouse respiratory system. Our findings confirmed that, by repeated administrations, rAs22U induces eosinophilic inflammation in the lung, in part by coordinating the production of both chemokines and cytokines necessary for the recruitment of eosinophils.
MATERIALS AND METHODSGeneration of rAs22U protein using the pET28a expression vectorFollowing confirmation of the PCR product sequences, the As22U clone was extracted for ligation into a pET28a expression vector system (Novagen, Darmstadt, Germany). Thereafter, ligates were transformed into E. coli strain BL21. After determining the optimal expression conditions, large-scale cell cultures were prepared via re-inoculation of overnight cultures of E. coli BL21 in 1 L of fresh lactose broth medium, containing 100 µg/ml of ampicillin, at a dilution factor of 1:100. The cells were cultured to an OD of 0.8-1.0 at A600 for approximately 8 hr, with vigorous agitation at 25℃. Induction of fusion protein expression was followed by the addition of isopropyl β-D-1-thiogalactopyranoside, at a final concentration of 0.1 mM. The rAs22U protein was purified using the HisTrap™ HP column (Amersham Biosciences, Little Calfont, UK). LPS was depleted from the rAs22U (i.e. endotoxin levels <0.01 µg/ml), using the Detoxi-Gel Affinity Pak prepacked columns (Amersham Biosciences), in accordance with the manufacturer's instructions.
Induction of the airway inflammatory reactionFemale C57BL/6 mice at the age of 5 weeks were purchased from Samtako Co. (Gyeonggi-do, Korea). The mice were bred in a SPF facility, at the Institute for Laboratory Animals of Pusan National University. Chicken egg OVA (Sigma, Seoul, Korea) was reconstituted in sterile PBS, at 1 mg/ml, and stored at -20℃. For intranasal challenge, 10 µl (10 µg) of rAs22U was added to 40 µl (40 µg) of OVA, immediately before intranasal administration. C57BL/6 mice were exposed to airway inflammation for 6 total challenges, as previously described [20-22]. One day after the last challenge, the lung function (i.e., airway hyperresponsiveness level) was evaluated, and then mice were sacrificed for analysis of the bronchial alveolar lavage fluid (BALF).
Airway hyperresponsiveness measurementsThe enhanced pause (PenH), a measure of bronchial constriction, was evaluated at baseline and after treatment with increasing doses of aerosolized methacholine (i.e., 2.5-50 mg/ml), via whole-body plethysmography for animals (Allmedicus, Korea). In the plethysmography procedure, the mice were allowed to acclimate for 3 min, exposed to nebulized saline for 4 min, then subsequently treated with increasing concentrations (0, 2.5, 5, 10, and 50 mg/ml) of nebulized methacholine (Sigma) in saline, using an ultrasonic nebulizer. After each nebulization, recordings were obtained for 3 min, and the PenH values measured during each 3 min period were averaged. Graphs of the PenH values, in response to the increasing methacholine concentrations, were constructed for each dose-matched group of mice.
Analysis of BALFBALF was isolated from mice after scarification with 200 µl of ketamine: lumpun: PBS (2:3:5) solution. The chest cavity was exposed to allow for expansion, then the trachea was carefully intubated and the catheter was secured with ligatures. Pre-warmed PBS was slowly inflated into the lungs and withdrawn. The collected BALF was then centrifuged, and the supernatants were maintained at -70℃, until used for ELISA. The cell pellets were resuspended and washed twice in PBS. After RBC lysis using the ACK lysis buffer (Invitrogen, Carlsbad, California, USA), the total number of cells was counted using a hemocytometer. The BALF cell smears were prepared with the Cytospin apparatus. The smears were then stained with Diff-Quik solution (Dade Diagnostics of Puerto Rico. Inc., Aguada, Puerto Rico) in order to determine the cell differentials, in accordance with conventional morphologic criteria.
Lung histopathologyHistological analyses were conducted, as described previously [23]. In brief, the lung tissues were fixed with formaldehyde and embedded in paraffin. The thin sections of the embedding tissues were then stained with hematoxylin-eosin stain. After staining, we microscopically examined the stained sections.
ELISAAnti-As22U specific immunoglobulins (IgG1, IgG2a, and IgE) in serum and cytokine interleukin levels (IL-4, IL-5, IL-13, and IL-17A) in BALF were determined via ELISA. All of these were conducted in accordance with the manufacturer's instructions (ebioscience, San Diego, California, USA). The absorbance of the final reactant was determined using an ELISA plate reader set at 450 nm.
The lung epithelial cell culture and rAs22U treatmentA mouse lung epithelial cell line (MLE12) was purchased from American Type Culture Collection. The cells were maintained in Dulbecco's Modified Eagle's Medium (Hyclone, Road Logan, Utah, USA) supplemented with 10% heat-inactivated FBS (Hyclone), 2 mM L-glutamine, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. The cells were then cultured at 37℃, in a humidified atmosphere containing 5% CO2. For rAs22U treatment (final concentration 1 µg/ml) the cells were plated in 10 cm plates at 1×106 cells/well and incubated for the indicated time periods. After 2 hr stimulation, cells were collected and lysed, and the total RNA was extracted using the QIAzol reagent (Qiagen Science, Valencia, California, USA).
RT-PCR analysisTotal RNA was generated to cDNA using moloney murine leukemia virus reverse transcriptase (Promega, Madison, Wisconsin, USA). For quantitation of both cytokine and transcription factor gene expression, cDNA samples were amplified in the iQ SYBR Green Supermix (Bio-Rad Laboratories Inc., Hercules, California, USA). The primer pairs used for RT-PCR are shown in Table 1.
StatisticsResults are represented as the mean±SD. Data are representative of at least 2 independent experiments performed with at least 4 mice in each group, unless otherwise indicated. Data were analyzed using the Student's t-test (n=2 groups). A 5% or lower P-value was considered to be statistically significant.
RESULTSrAs22U could elicit airway allergic inflammationTo investigate the role of rAs22U as an allergen, we repeatedly applied rAs22U with OVA at increasing doses into the nose of mice and evaluated the allergic responses elicited in the lung. After 6 times challenges of rAs22U with OVA, we found evident immune cell infiltrations, particularly eosinophils, into the lung (Fig. 1A). In addition, we noted infiltration of inflammatory cells in the pulmonary parenchyma of the mice (Fig. 1B). These influxes of inflammatory cells induced severe inflammation and pulmonary edema of the alveolar wall. Both bronchial epithelial cell and goblet cell hyperplasia were observed in the lung of mice treated with rAs22U and OVA. In order to determine whether the lung inflammation induced by rAs22U was associated with alterations in the lung function, variations in intra-tracheal pressure in response to increasing methacholine doses were evaluated. The values of Penh after methacholine administration (dose ranging from 2.5 mg/ml to 50 mg/ml) increaed significantly in the mouse group treated with rAs22U and OVA, as compared with the mice treated only with OVA (Fig. 1C).
rAs22U elevated Th2 and Th17 productions in the lungIn order to determine the manner in which rAs22U could influence cytokine secretion in BALF, ELISA was performed to detect IL-4, IL-5, IL-13, and IL-17A cytokine levels. Concentrations of these cytokine increased significantly in the BALF of rAs22U treated mice (Fig. 2). These findings show that rAs22U could elicit allergic airway reactions, via activating Th17 and Th2 cytokines. Additionally, rAs22U elicited a significant increase in the OVA-specific IgE and IgG1 serum levels, although the production of OVA-specific IgG2a remained unaffected (Fig. 3). These results showed that rAs22U could increase immune response allergen-specific antibody production.
rAs22U activated CXCL1 and allergy specific genes expressionIn order to investigate the mechanisms of both Th2 and Th17 elevation by As22U, we evaluated several cytokine and chemokine gene expression levels in the MLE12 after rAs22U treatment. The results demonstrated that Gro-α (CXCL1) gene expression levels increased at 2 hr after As22U treatment. After 6 hr, thymus-and-activation-regulated-chemokine (TARC, CCL17) and eotaxin (CCL11) gene expression reached the highest levels. Although IL-25 gene expression increased significantly at 2 hr after rAs22U treatment, the increase was smaller than for other genes (Fig. 4).
DISCUSSIONRecently, much interest has focused on the relationship between helminth infections and atopic diseases by many researchers. This interest is mainly because these 2 disorders share immunological features, such as the dominant Th2 type of immune responses, which is characterized by increased serum IgE and eosinophilia [24]. Remarkably, certain helminth infections have been reported to suppress [25-27], while others to contribute to the development of allergic manifestations [2,3,28,29]. Most parasites that involved in allergic inflammations were larval stage nematodes, especially roundworms in several mammals. Roundworms have been found to infect most mammals, and also exhibit host specificity. The majority of roundworms generally show a visceral larva migration period during their life cycle, which is essential for their development into adult worms. Many case reports have demonstrated that roundworm larvae can cause asthma, pneumonia, and airway inflammation [30-32]. In addition, parasite-derived protein could elicit allergic responses [33-35].
In our study, we evaluated the possibility of As22U to act as an allergen, using the new allergic mouse model. As results, repeated treatments with rAs22U could elicit strong airway inflammation, with high eosinophilia and IgE levels in experimental mice (Figs. 1, 3). In addition, we found significantly increased IL-4 and IL-5 cytokine levels in BALF (Fig. 2). It is well known that several Th2 cytokines, such as IL-4, IL-5, IL-9, and IL-13, induced by Anisakis infection and also play important roles in development of airway hyperreactivity [36]. Especially IL-4 regulates the isotype class switching in B cells to IgE synthesis, and IL-5 stimulates the eosinophil growth, activates these cells, and prolongs the eosinophil survival [37]. Eosinophils and IgE proved vitally important in the allergy-induced Th2 responses, and also in increasing the number of these cells. The increased serum levels of IgE were specific responses to parasite infections and this response was shown to be elicited only by treatment with parasite total proteins [38,39].
Interestingly, we also found that the IL-17A cytokine level in BALF was significantly increased (Fig. 2). In addition, rAs22U treatment could activate CXCL1 gene expression in the mouse lung epithelial cell (Fig. 4). These results showed that rAs22U could elicit allergic airway inflammation via both Th17 and Th2 cell activations. In our previous study, repeated treatments with the excretory-secretory protein extracted from A. simplex could elicit neutrophil recruitment and IL-17A production, which might play a critical role in the Anisakis-associated allergic reaction [33]. IL-17A and IL-17F cytokines are members of the IL-17 family, playing central roles in allergic inflammations. Recent studies have reported that IL-17A and IL-17F cytokine production from a distinct Th lymphocyte subset (i.e. Th17) was specifically induced by IL-23, which was generated by both dendritic cells and macrophages, in response to microbial stimuli. The IL-23/IL-17 axis may therefore constitute a link between infections and allergic diseases [40-42]. In addition, IL-17A, IL-17F, and IL-23 cytokines have been shown to induce the release of chemokines Gro-α (CXCL1), IL-8 (CXCL8), and MIP-1beta (CCL4) from eosinophils [42]. Shainheit et al. [43] previously reported that schistosome egg-stimulated dendritic cells plus naive CD4 T-cells from CBA mice resulted in increased levels of pre-inflammatory cytokines, as well as IL-17, and the chemokines CXCL1, CXCL2, and CCL2. They demonstrated that after neutralization of IL-23 and IL-1, but not of IL-6 or IL-21, egg-induced IL-17A production was profoundly inhibited. They also emphasized that parasite recognition, followed by a genetically determined innate proinflammatory response, induces the development of Th17 cells, and thus controls the outcome of immunopathology in schistosomiasis [43].
We evaluated alteration of allergy-specific chemokine gene (i.e. TARC and eotaxin) expressions by rAs22U treatment, and found that the gene expressions were significantly increased after the treatment (Fig. 4). Th2 cells preferentially express several receptors, such as CCR3 (receptor of eotaxin, CCL13, and CCL28), CCR4 (receptor of TARC and MDC), and CCR8 (receptor of CCL1), and the chemokines active on these receptors are increased in allergic inflammation, making them attractive candidates to mediate Th2 cell-specific recruitment [44,45]. Either inhibition or deletion of single chemokines or chemokine receptors (e.g., eotaxin, MDC, TARC) have not abolished the Th2 cell recruitment in allergic pulmonary inflammations [44]. Therefore, rAs22U might elicit Th2 cell recruitment via elevation of both eotaxin and TARC secretion levels in the lung epithelial cell. In conclusion, rAs22U may induce allergic airway inflammation as the result of enhanced Th2 and Th17 responses.
ACKNOWLEDGMENTThis study was supported by Medical Research Institute Grant (2007-6), Pusan National University Hospital.
REFERENCES1. Moreno-Ancillo A, Caballero MT, Cabanas R, Contreras J, Martin-Barroso JA, Barranco P, Lopez-Serrano MC. Allergic reactions to Anisakis simplex parasitizing seafood. Ann Allergy Asthma Immunol 1997;79:246-250. PMID: 9305232.
2. Nieuwenhuizen N, Lopata AL, Jeebhay MF, Herbert DR, Robins TG, Brombacher F. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J Allergy Clin Immunol 2006;117:1098-1105. PMID: 16675338.
3. Armentia A, Lombardero M, Callejo A, Martin Santos JM, Gil FJ, Vega J, Arranz ML, Martinez C. Occupational asthma by Anisakis simplex. J Allergy Clin Immunol 1998;102:831-834. PMID: 9819301.
4. Barbuzza O, Guarneri F, Galtieri G, Gangemi S, Vaccaro M. Protein contact dermatitis and allergic asthma caused by Anisakis simplex. Contact Dermatitis 2009;60:239-240. PMID: 19338604.
5. Jeebhay MF, Robins TG, Lehrer SB, Lopata AL. Occupational seafood allergy: A review. Occup Environ Med 2001;58:553-562. PMID: 11511741.
6. Nieuwenhuizen N, Herbert DR, Brombacher F, Lopata AL. Differential requirements for interleukin (IL)-4 and IL-13 in protein contact dermatitis induced by Anisakis. Allergy 2009;64:1309-1318. PMID: 19254288.
7. Moneo I, Caballero ML, Gomez F, Ortega E, Alonso MJ. Isolation and characterization of a major allergen from the fish parasite Anisakis simplex. J Allergy Clin Immunol 2000;106:177-182. PMID: 10887322.
8. Perez-Perez J, Fernandez-Caldas E, Maranon F, Sastre J, Bernal ML, Rodriguez J, Bedate CA. Molecular cloning of paramyosin, a new allergen of Anisakis simplex. Int Arch Allergy Immunol 2000;123:120-129. PMID: 11060483.
9. Asturias JA, Eraso E, Martinez A. Cloning and high level expression in Escherichia coli of an Anisakis simplex tropomyosin isoform. Mol Biochem Parasitol 2000;108:263-267. PMID: 10838230.
10. Rodriguez-Mahillo AI, Gonzalez-Munoz M, Gomez-Aguado F, Rodriguez-Perez R, Corcuera MT, Caballero ML, Moneo I. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int J Parasitol 2007;37:907-917. PMID: 17324433.
11. Kobayashi Y, Ishizaki S, Shimakura K, Nagashima Y, Shiomi K. Molecular cloning and expression of two new allergens from Anisakis simplex. Parasitol Res 2007;100:1233-1241. PMID: 17180690.
12. Kobayashi Y, Shimakura K, Ishizaki S, Nagashima Y, Shiomi K. Purification and cDNA cloning of a new heat-stable allergen from Anisakis simplex. Mol Biochem Parasitol 2007;155:138-145. PMID: 17689675.
13. Rodriguez-Perez R, Moneo I, Rodriguez-Mahillo A, Caballero ML. Cloning and expression of Ani s 9, a new Anisakis simplex allergen. Mol Biochem Parasitol 2008;159:92-97. PMID: 18378015.
14. Caballero ML, Umpierrez A, Moneo I, Rodriguez-Perez R. Ani s 10, a new Anisakis simplex allergen: Cloning and heterologous expression. Parasitol Int 2011;60:209-212. PMID: 21310242.
15. Kobayashi Y, Ohsaki K, Ikeda K, Kakemoto S, Ishizaki S, Shimakura K, Nagashima Y, Shiomi K. Identification of novel three allergens from Anisakis simplex by chemiluminescent immunoscreening of an expression cDNA library. Parasitol Int 2011;60:144-150. PMID: 21262386.
16. Rodriguez-Mahillo AI, Gonzalez-Munoz M, Moneo I. Identification and allergenic characterisation of a new isoform of the A. simplex allergen Ani s 4. Mol Biochem Parasitol 2008;160:152-156. PMID: 18501441.
17. Park JS, Cho MK, Yu HS, Ahn SC. Identification of 24 kDa excretory secretory protein of Anisakis simplex. Experimental Parasitology 2012;130:69-72. PMID: 22001446.
18. Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol 2009;183:1427-1434. PMID: 19561109.
19. Chou H, Tam MF, Lee LH, Chiang CH, Tai HY, Panzani RC, Shen HD. Vacuolar serine protease is a major allergen of Cladosporium cladosporioides. Int Arch Allergy Immunol 2008;146:277-286. PMID: 18362473.
20. Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol 2007;120:334-342. PMID: 17544098.
21. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 2007;204:1509-1517. PMID: 17562814.
22. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 2007;204:1837-1847. PMID: 17635955.
23. Trujillo-Vargas CM, Mayer KD, Bickert T, Palmetshofer A, Grunewald S, Ramirez-Pineda JR, Polte T, Hansen G, Wohlleben G, Erb KJ. Vaccinations with T-helper type 1 directing adjuvants have different suppressive effects on the development of allergen-induced T-helper type 2 responses. Clin Exp Allergy 2005;35:1003-1013. PMID: 16120081.
24. Falcone FH, Loukas A, Quinnell RJ, Pritchard DI. The innate allergenicity of helminth parasites. Clin Rev Allergy Immunol 2004;26:61-72. PMID: 14755076.
25. Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 2009;126:3-11. PMID: 19120493.
26. Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol 2009;39:457-464. PMID: 18835272.
27. Pacifico LG, Marinho FA, Fonseca CT, Barsante MM, Pinho V, Sales-Junior PA, Cardoso LS, Araujo MI, Carvalho EM, Cassali GD, Teixeira MM, Oliveira SC. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: A major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect Immun 2009;77:98-107. PMID: 18824533.
28. Pinelli E, Brandes S, Dormans J, Gremmer E, van Loveren H. Infection with the roundworm Toxocara canis leads to exacerbation of experimental allergic airway inflammation. Clin Exp Allergy 2008;38:649-658. PMID: 18167123.
29. Buijs J, Egbers MW, Lokhorst WH, Savelkoul HF, Nijkamp FP. Toxocara-induced eosinophilic inflammation. Airway function and effect of anti-IL-5. Am J Respir Crit Care Med 1995;151:873-878. PMID: 7881685.
31. Takeuchi H, Zaman K, Takahashi J, Yunus M, Chowdhury HR, Arifeen SE, Baqui A, Wakai S, Iwata T. High titre of anti-Ascaris immunoglobulin E associated with bronchial asthma symptoms in 5-year-old rural Bangladeshi children. Clin Exp Allergy 2008;38:276-282. PMID: 18070165.
32. Sakakibara A, Baba K, Niwa S, Yagi T, Wakayama H, Yoshida K, Kobayashi T, Yokoi T, Hara K, Itoh M, Kimura E. Visceral larva migrans due to Ascaris suum which presented with eosinophilic pneumonia and multiple intra-hepatic lesions with severe eosinophil infiltration--outbreak in a Japanese area other than Kyushu. Intern Med 2002;41:574-579. PMID: 12132528.
33. Cho MK, Ahn SC, Kim DH, Yu HS. Parasite excretory-secretory proteins elicit TRIF dependent CXCL1 and IL-6 mediated allergic inflammation. Parasite Immunol 2010;32:354-360. PMID: 20500664.
34. Marsland BJ, Camberis M, Le Gros G. Secretory products from infective forms of Nippostrongylus brasiliensis induce a rapid allergic airway inflammatory response. Immunol Cell Biol 2005;83:40-47. PMID: 15661040.
35. Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen-induced inflammatory responses. Chem Immunol Allergy 2006;90:45-64. PMID: 16210902.
36. Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol 2000;165:108-113. PMID: 10861042.
37. Sher A, Coffman RL, Hieny S, Cheever AW. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol 1990;145:3911-3916. PMID: 2123226.
38. Ishizaka T, Urban J Jr, Takatsu K, Ishizaka K. Immunoglobulin E synthesis in parasite infection. J Allergy Clin Immunol 1976;58:523-538. PMID: 1085781.
39. Ehigiator HN, Stadnyk AW, Lee TD. Extract of Nippostrongylus brasiliensis stimulates polyclonal type-2 immunoglobulin response by inducing De novo class switch. Infect Immun 2000;68:4913-4922. PMID: 10948105.
40. Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol 2003;462:193-198. PMID: 12591113.
41. Kawaguchi M, Kokubu F, Matsukura S, Ieki K, Odaka M, Watanabe S, Suzuki S, Adachi M, Huang SK. Induction of C-X-C chemokines, growth-related oncogene alpha expression, and epithelial cell-derived neutrophil-activating protein-78 by ML-1 (interleukin-17F) involves activation of Raf1-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase 1/2 pathway. J Pharmacol Exp Ther 2003;307:1213-1220. PMID: 14557379.
42. Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol 2008;180:5625-5635. PMID: 18390747.
43. Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol 2008;181:8559-8567. PMID: 19050275.
44. Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med 2001;193:1087-1096. PMID: 11342593.
45. Sallusto F. The role of chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Haematologica 1999;84(Suppl EHA-4):28-31. PMID: 10907461.
Fig. 1Induction of eosinophilic airway inflammation after rAs22U treatment. (A) The differential immune cells were counted in BALF. The number of immune cells, especially eosinophils and lymphocytes were increased in BALF of mice treated with rAs22U. (B) A thin section of the lung stained with hematoxylin-eosin (Bar=50 µm, ×100). In the lungs of mice treated with rAs22U, massive peribronchial infiltration with immune cells and hyperplasis of bronchial epithelial cells were observed. Also, thickened bronchial epithelial cells were observed in rAs22U-treated mice. (C) Enhanced pause (PenH) was increased at baseline and after treatment with increasing doses of aerosolized methacholine (0 to 50 mg/ml). The PenH value of rAs22U-treated mice was significantly higher than that of the only OVA-treated mice (*, P<0.05; **, P<0.01). 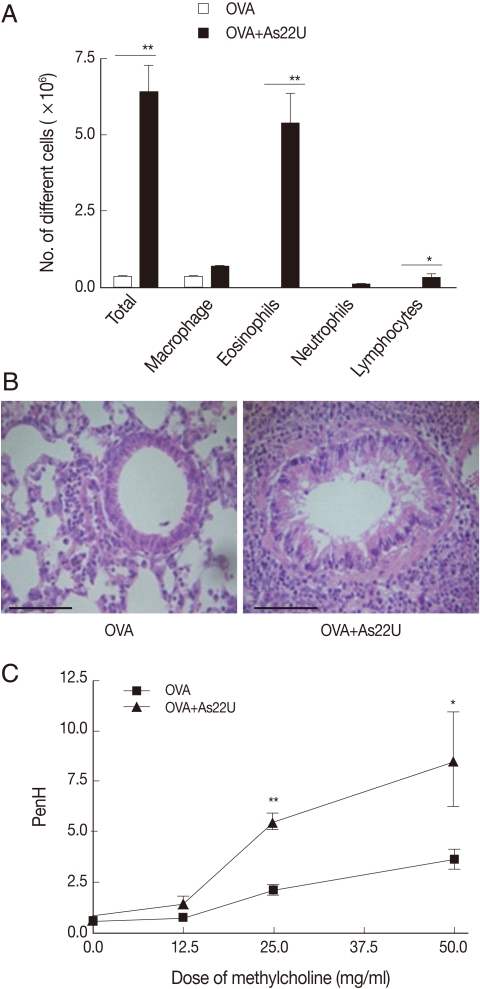
Fig. 2Cytokine levels in BALF. The level of IL-4, IL-5, IL-13, and IL-17A in BALF were determined using sandwich ELISA. Levels of all cytokines were significantly increased after rAs22U treatment. The data for cytokine levels in the BALF are expressed as means±SD of values from individual mice (**, P<0.01; ***, P<0.001). 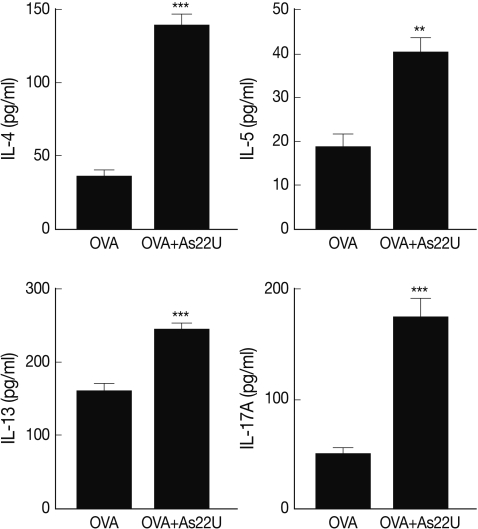
Fig. 3The level of OVA-specific immunoglobulin in serum. OVA-specific IgE, IgG1, and IgG2a levels in serum were measured. The 96-well plates were incubated with OVA (final concentration 10 µg/ml) for 16 hr at 4℃. After blocking with 1% BSA, sera ere diluted with PBS to 1:104-106 for IgG1 and IgG2a measurement, and the levels of immunoglobulins were measured. The rAs22U treated mice had significantly increased serum levels of OVA-specific IgE and IgG1 (**, P<0.01; ***, P<0.001). 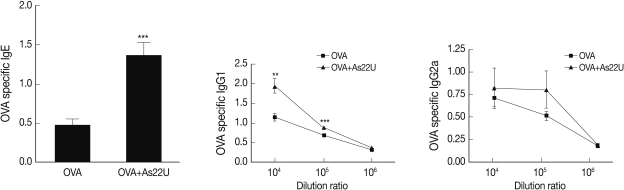
Fig. 4Chemokine gene expression in mouse lung epithelial cells after rAs22U treatment. After treatment with 10 µg/ml (final concentration) rAs22U to MLE12 cells, the expression of several chemokine genes was evaluated every 2 hr until 10 hr after treatment (*, P<0.05; **, P<0.01, compare to the level of gene expression at 0 hr). 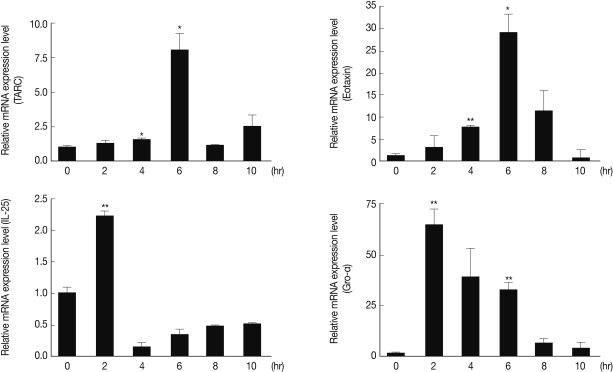
Table 1.Primers used for real-time PCR
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||