Abstract
Babesia spp. were detected from 4 asymptomatic pukus captured on a game ranch in central Zambia in October 2008. Blood smears were examined in 4 species of aymptomatic free-ranging antelopes, namely the puku (Kobus vordanii), reedbuck (Redunca arundinum), bushbuck (Tragelaphus sylvaticus), and kudu (Tragelaphus strepsiceros), and showed the presence of Babesia parasites only in the puku. In the puku, the prevalence of babesiosis was estimated at 33.3% (n=12), while the overall prevalence in all examined animals was 8.5% (n=47). The parasites showed morphological characteristics of paired ring-like stages with the length varying between 1.61 µm and 3.02 µm (mean=2.12 µm, n=27; SD=0.76 µm). Both the infected and non-infected pukus showed good body condition scores (BCS), while the dominant tick species detected from all animals were Rhipicephalus appendiculatus, Rhipicephalus spp., and Boophilus spp. To our knowledge this is the first report of Babesia spp. infection in pukus in Zambia. These findings suggest that wildlife could play an important role in the epidemiology of babesiosis in Zambia.
Pukus (Kobus vardoni) are small to medium-sized antelopes belonging to the subfamily Reduncinae under the family Bovidae [1]. They are about 80 cm tall and weigh between 62-74 kg with an average weight of 68 kg. They are generally between 1.5-1.7 m long while the horn length for the males averages 54 cm. Pukus used to occupy large areas of open savannah in south-central Africa. Now the population has declined to near threatened species with largest populations being present in Tanzania and Zambia, while numbers in Malawi, Botswana, Democratic Republic of Congo, and Angola have declined tremendously [2-7]. Unlike lechwes (Kobus leche) which are semi-aquatic, pukus utilize moist savannah and floodplain as well as narrow stretches of grasslands lying between water sources and woodland areas [1,5].
As a result of the population declining, there has been a deliberate shift in policy to promote ex-situ conservation of this species with approximately 42 game ranches keeping pukus in Zambia. However, one of the greatest constraints to the expansion of ex-situ conservation is the introduction of wildlife into territories endemic with livestock diseases that have the potential to cause clinical diseases in susceptible wildlife species. In Zambia, tick-borne diseases are one of the greatest challenges to livestock production [8], which has led most cattle ranchers to switch to game ranching. Although it is generally perceived that wildlife are resistant to tick-borne diseases, risk factors emanating from transmission of wildlife diseases to livestock are often overestimated. On the other hand, it is important to determine the impact of diseases of livestock origin on wildlife introduced in areas previously occupied by livestock. This would provide more effective advice to ranchers who are switching from cattle to game ranching. Hence, in the present study, we examined different antelope species on a game ranch that used to be a cattle ranch for the presence of ticks and tick-borne infections, in particular, babesiosis.
The study was carried out in October 2008 in central Zambia on a game ranch which used to be a cattle ranch 12 years prior to this study. The game ranch is endowed with several antelope species. As shown in Table 1, 4 animal species captured for translocation were used in the study. At capture, animals were immobilized using M99 (etorphine hydrochloride, Norvatis, Johannesburg, South Africa). The body condition score (BSC) for each animal was determined by physical inspection. Emaciated animals with little muscle and no fat deposits on body surfaces with the skin of the neck falling slowly or staying longer in a fold after the pliability test were scored as poor (-). Moderately fat animals with the skin of the neck falling moderately faster than those of the poor BCS were scored fair (+) while animals with a lot of fat with the skin falling faster to its original state after the pliability test were scored as good (++).
Blood smears were made by puncturing the ear vein soon after immobilization. Ticks were collected and stored in 70% alcohol for identification. Once all samples had been collected and BCSs determined, animals were revived using M5050 revivon (dreprenorphine, Norvatis, Johannesburg, South Africa). At the School of Veterinary Medicine, which is based at University of Zambia, slides were stained using Giemsa stain and were examined for the presence of blood parasites under a light microscope. The observed blood parasites were identified as Babesia spp. based on morphological characteristics (Fig. 1) [9]. As pointed out by others [10,11] that the presence of pairs or tetrads also known as 'Maltese cross' in stained erythrocytes is diagnostic for babesiosis, we detected the presence of pairs of ring-like Babesia organisms as shown in Fig. 1, although other growth stages were also seen. The length of the parasites varied between 1.61 µm and 3.02 µm with an average of 2.12 µm (n=27; SD=0.76 µm). Similarly, ticks were identified using standard keys as described elsewhere [9]. Body condition scores are shown in Table 1. Babesia parasites were detected only in 4 pukus (Table 1) giving a species prevalence of 33.3% (n=12), while the overall prevalence for all examined animals was 8.5% (n=47).
To our knowledge this is the first report of Babesia spp. infection in pukus in Zambia. Although the infection was detected only in pukus (Table 1), we could not rule out the possibility of other animals being infected because we did not use other diagnostic tests, such as molecular biology based tools that are more sensitive to detect the presence of parasites in animals that had low infection rates not easy to detect on blood smears. On the other hand, use of serological assays, such as ELISA or indirect flourescent antibody tests (IFAT), could have been used to determine the seroprevalence of animals previously exposed to the disease but not having active infections at the time of sampling. Suffice to mention that experimental studies have shown differences in susceptibility to different Babesia spp. within the Bovidae family. For example, Karbe et al. [12] showed that 2 elands (Taurotragus oryx) inoculated with Babesia bigemina obtained from infected cattle did not express the parasite in their blood, and the parasite was not detected after splenectomizing 1 of the animals. In another experiment, Karbe et al. [12] established a carrier state in African buffaloes (Syncerus caffer) injected with B. bigemina obtained from cattle. Hence, it is likely that the absence of Babesia organisms in other animal species (Table 1) was indicative of that these animals were not susceptible to the Babesia spp. detected in the puku. However, there is need for more detailed studies to verify these assertions.
Given that BCSs obtained in Table 1 were good for both the infected and non-infected pukus, these findings suggest that Babesia parasites in pukus did not cause clinical diseases, although we did not carry out hematological tests to determine whether these parasites caused changes in blood parameters of the infected animals. Consistent with our observations, Penzhorn [13] suggested that the occurrence of Babesia parasites in wildlife is often an incidental finding and that affected animals do not often present clinical diseases. Observation made by several scientists show that clinical babesiosis in wildlife is often stress-related leading to mortality in some cases [13-17]. For example, Martinaglia [15] reported clinical babesiosis in a sable antelope (Hippotragus niger) that was translocated to a zoo in South Africa. The animal died of stress-related babesiosis due to adaptation to the new environment in the zoo. Similarly, babesiosis-related mortalities caused by stress of translocation have been reported in Black rhinoceros (Diceros bicornis) in Kenya and Tanzania [14,16].
Although we report here the first case of Babesia infection in pukus in Zambia, Babesia bovis and B. bigemina have been reported to cause red-water disease in cattle for a long time in Zambia [8,18,19]. As pointed out by Jongeian et al. [19], the epidemiology of babesiosis in cattle in Zambia is highly influenced by the distribution and life cycle of the vector ticks. It is likely that these ticks have a similar influence on the occurrence and seasonal variations of Babesia infections in wildlife. It is interesting to note that the first report of B. bovis, a species infecting bovids, was first reported from central Zambia [18] in the same area where the present finding of Babesia spp. in the puku was detected. Given that various tick species involved in the transmission of Babesia parasites have been present in the area for a long time, it is likely that these tick-borne diseases have been endemic in this area for a long time. Hence, introducing wildlife in such ecosystems exposes wild animals to tick-borne infections. However, there is need to develop molecular biologic tools for characterizing different Babesia parasites infecting wildlife and to develop a trace back system for monitoring the transmission dynamics of Babesia infections between cattle and wildlife in Zambia. Future studies should determine the role of wildlife in the persistence of babesiosis in Zambia.
REFERENCES1. Zylstra J. Kobus vardonii (On-line). Animal diversity web. 2002. cited 2011 Sept. Available from: http://animaldiversity.ummz.edu/site/accounts/information/kobus_vardonii.html
2. Dipotso FM, Skarpe C. Population status and distribution of puku in a changing riverfront habitat in northern Botswana. South African J Wildlife Res 2006;36:89-97.
3. Goldspink CR, Holland RK, Sweet G, Stjernstedt R. A note on the distribution and abundance of puku, Kobus vardoni Livingstone, in Kasanka National Park, Zambia. Afr J Ecol 1998;36:23-33.
4. Jenkins RK, Maliti HT, Corti GR. Conservation of the puku antelope (Kobus vardoni, Livingstone) in the Kilombero Valley, Tanzania. Biodivers Conserv 2003;12:787-797.
5. Nowak R. Walker's mammals of the world online. 1995. cited 2011 Sept. Available from: http://www.press.jhu.edu/books/walker/artiodactyla.bovidae.kobus.html
7. Rosser AM. Environmental and reproductive seasonality of puku, Kobus vardonii, in Luangwa-Valley, Zambia. Afr J Ecol 1989;27:77-88.
8. Makala LH, Mangani P, Fujisaki K, Nagasawa H. The current status of major tick borne diseases in Zambia. Vet Res 2003;34:27-45. PMID: 12588682.
9. Soulsby E. Helminth, Arthropod and Protozoa of Domestic Animals. 1982, London, UK. Bailliere, Tindall and Cassell.
10. Schuster FL. Cultivation of Babesia and Babesia-like blood parasites: Agents of an emerging zoonotic disease. Clin Microbiol Rev 2002;15:365-373. PMID: 12097245.
11. Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev 2000;13:451-469. PMID: 10885987.
12. Karbe E, Grootenhuis JG, Kelley S, Karstad L. Experiments on the Babesia bigemina carrier state in East African buffalo and eland. Tropenmed Parasitol 1979;30:313-317. PMID: 543000.
13. Penzhorn BL. Babesiosis of wild carnivores and ungulates. Vet Parasitol 2006;138:11-21. PMID: 16500026.
14. Brocklesby D. Babesia species of the Black rhinoceros. Vet Rec 1967;80:484.
15. Martinaglia G. Red water (babesiosis) in sable antelope. J S Afr Vet Med Assoc 1930;1:41-42.
16. McCullogh B, Achard PL. Mortalities assocaited with capture, translocation, trade and exhibition of black rhinoceros. Int Zool Ybk 1969;9:184-191.
17. McInnes EF, Stewart CG, Penzhorn BL, Meltzer DG. An outbreak of babesiosis in imported sable antelope (Hippotragus niger). J S Afr Vet Assoc 1991;62:30-32. PMID: 2051446.
18. Jongejan F, Lemche J, Mwase ET, Kafunda MM. Bovine babesiosis (Babesia bovis Infection) in Zambia. Vet Q 1986;8:168-171. PMID: 3727339.
19. Jongejan F, Perry BD, Moorhouse PDS, Musisi FL, Pegram RG, Snacken M. Epidemiology of bovine babesiosis and anaplasmosis in Zambia. Trop Anim Health Prod 1988;20:234-242. PMID: 3070875.
Fig. 1Pairs of Babesia parasites (ring-like organisms) in the erythrocytes of a puku designated as A, while B shows Babesia parasites at different stages of the growth cycle. 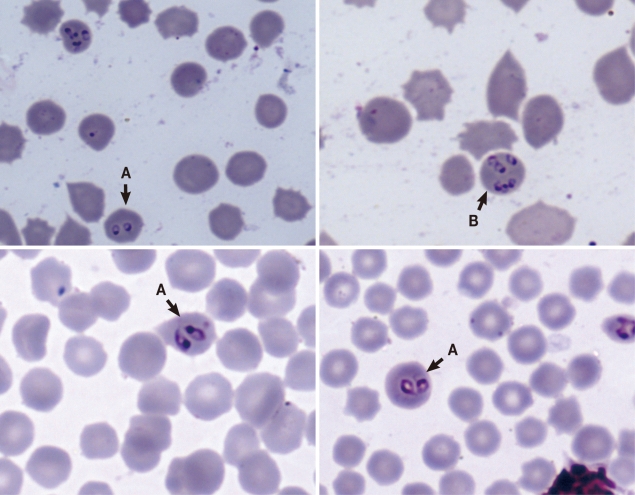
Table 1.Animal species examined for the presence of blood parasites |
|
||||||||||||||||||||||||||||||||||||