Chai, Sohn, Na, and Van De: Echinostoma revolutum: Metacercariae in Filopaludina Snails from Nam Dinh Province, Vietnam, and Adults from Experimental Hamsters
Abstract
We detected metacercariae of Echinostoma revolutum in Filopaludina sp. snails purchased from a local market in Nam Dinh Province for the first time in Vietnam. Adult flukes were harvested from experimentally infected hamsters at days 14 and 17 post-infection. The metacercariae were round, 170-190 µm (n=15) in diameter, with a cyst wall thickness of about 12 µm. A total of 37 collar spines were arranged around the head collar, and large excretory granules were seen in 2 canals of the excretory bladder. The 14-day old adult flukes were elongated, ventrally curved, and 5.0-7.2×0.8-1.3 mm (n=20). The head collar had a total of 37 collar spines arranged in 2 alternating rows, including 5 corner spines on each side. The cirrus sac contained a saccular seminal vesicle, a prostatic gland, and an unarmed cirrus. Two tandem testes were smooth or slightly lobed. Eggs were ovoid to elliptical, 110-118×70-75 µm. These morphological characters were similar to those of E. revolutum and E. jurini. We tentatively identified it as E. revolutum because the validity of E. jurini remains to be elucidated. The taxonomic relationship of E. revolutum and E. jurini is discussed.
Key words: Echinostoma revolutum, Echinostoma jurini, echinostome, metacercaria, Filopaludina snail, Vietnam
Echinostomes (=family Echinostomatidae) are intestinal flukes of birds and mammals, including humans [ 1, 2]. In heavy infections, they can cause mucosal ulceration, bleeding, and severe inflammation in human patients. To date, 20 species of zoonotic echinostomes belonging to 9 genera have been discovered worldwide ( Echinostoma, Echinochasmus, Acanthoparyphium, Artyfechinostomum, Echinoparyphium, Episthmium, Himasthla, Hypoderaeum, and Isthmiophora) [ 1]. Among them, Echinostoma is the most important and 7 zoonotic species of Echinostoma are known; Echinostoma revolutum, E. angustitestis, E. cinetorchis, E. echinatum, E. hortense, E. ilocanum, and E. macrorchis [ 1].
E. revolutum (Froelich, 1802) Looss, 1899 was the first echinostome species described in the literature [ 1]. It was first discovered as an intestinal fluke of ducks but is now known to infect a variety of avian and mammalian species, including geese, muskrats, and humans [ 1- 3]. E. revolutum is found worldwide, including Asia, Europe, Africa, Australia, New Zealand, and the Americas [ 1]. In Asia, E. revolutum has been reported from China, Taiwan, Korea, Japan, Indonesia, Malaysia, Thailand, India, Russia, and Cambodia [ 1- 6]. Vietnam was among the list of countries where E. revolutum was distributed [ 6]. However, it was based upon a suggestion on the presence of the larval stage, Cercaria spinifera [ 7]. No other detailed information about this echinostome in Vietnam is available. In the present study, we purchased Filopaludina sp. snails from a local market in Nam Dinh Province, Vietnam and examined them for the presence of echinostome metacercariae. We detected metacercariae and identified them as E. revolutum through recovery of adult flukes from experimental hamsters.
Filopaludina sp. snails ( Fig. 1) were purchased from a local market in Nam Dinh Province, Vietnam in April 2005 and transferred to our laboratory (Gyeongsang National University, Jinju, the Republic of Korea) on ice. They (n=30) were divided into 6 groups, crushed with their shells using a mortar and pestle, and digested for 2 hr in pepsin-HCl solution. A total of 450 metacercariae from 30 snails (15 per snail) were collected in the digested material using a stereomicroscope. Metacercariae were examined morphologically and measured using a light microscope. Three ducks were orally infected with 100 metacercariae each and 2 hamsters were orally infected with 50 metacercariae each. A total of 35 adult flukes were recovered from the small intestine of the 2 hamsters (average recovery; 35%) at days 14 and 17 post-infection (PI). No worms were recovered from the the ducks. Twenty adult worms were fixed with 10% neutral buffered formalin under coverslip pressure, stained with Semichon's acetocarmine, and measured using a light microscope.
To observe the surface ultrastructure, some of the adult worms were washed several times with 0.2 M cacodylate buffer (pH 7.2) and fixed with 2.5% glutaraldehyde at 4℃. After washing 3 times with the buffer, they were dehydrated through a graded ethanol series (50%, 70%, 80%, 90%, 95%, and absolute alcohol), dried in a critical point dryer, coated with gold (JFC-1100E ion sputtering device), and examined with a scanning electron microscope (SEM) (Philips XL-30S) at an accelerating voltage of 20 kV.
The metacercarial cyst was almost spherical and 170-190 µm in diameter, with a thick outer wall of about 12 µm and a thin inner opaque wall of about 3 µm ( Fig. 2). They had characteristically large, round excretory granules in 2 descending canals of the main excretory bladder. Collar spines, 37 in total, were clearly visible around the head collar, with 5 corner spines on each side and 27 ventral, lateral, and dorsal spines, in 2 alternating rows ( Fig. 2). The metacercariae were detected mainly in the pericardial sac of the snail. The adult flukes were elongated, ventrally curved, and 5.0-7.2×0.8-1.3 mm (n=20) ( Fig. 3). Eggs were operculated, elliptical, with a small abopercular wrinkle or thickening, and 110-118×70-75 µm (n=20) ( Fig. 4). The head collar of the adult was distinct, bearing 37 collar spines arranged in 2 alternating rows, including 5 corner spines on each side. The oral sucker was subterminal. The prepharynx was very short, and the pharynx was well developed. The esophagus was somewhat long. The cirrus sac was well developed, containing a saccular seminal vesicle and an unarmed cirrus. The ventral sucker was round and moderately large. The ovary was transversely elliptical and located on the median line of the body. Testes were tandom, smooth or slightly lobed. In SEM observations, the adult flukes were elongated and leaf-like, with a distinct head collar and oral sucker anteriorly, and a ventrally protruding ventral sucker near the anterior 1/5 region of the body ( Figs. 5- 7). The tegument of the anterior region of the body was densely covered with scale-like spines ( Figs. 5- 7). The tegumental spines became sparse toward the posterior part of the body. There were numerous ciliated or aciliated sensory papillae distributed around the oral sucker and head collar ( Fig. 6). The arrangement of the collar spines, including the 5 corner spines, was clearly visible. The corner spines were characteristically arranged in 2 rows, 3 oral and 2 aboral, on each side ( Fig. 6). Small, scale-like tegumental spines were distributed on the surface around the ventral sucker ( Fig. 7). On the lip of the ventral sucker, many sensory papillae were distributed. The cirrus was seen to have protruded out of the cirrus sac. The results of this study indicated that the life cycle of E. revolutum is mainained in Vietnam, using Filopaludina snails or other species of freshwater molluscs or amphibians as the second intermediate host. Eating raw or improperly cooked snails may cause human echinostomiasis which should be verified through epidemiological surveys and clinical interventions in Vietnam.
There was a taxonomic problem in identifying our specimens because of the complexity in the systematics of 37-collar-spined echinostomes, the so-called ' revolutum' group [ 8, 9]. Within this group, more than 30 species have been described. However, at present, only 12 species, E. revolutum (Froelich, 1802) [ 1, 6, 9], E. caproni Richard, 1964 [ 9- 11], E. cinetorchis Ando and Ozaki, 1924 [ 1, 12]; E. deserticum Kechemir et al., 2002 [ 9, 13], E. echinatum (Zeder, 1803) [ 14- 16], E. friedi Toledo et al., 2000 [ 9, 17], E. jurini (Skvortsov, 1924) [ 9, 18], E. luisreyi Maldonado et al., 2003 [ 9, 10], E. miyagawai Ishii, 1932 [ 9, 14], E. paraensei Lie and Basch, 1967 [ 9, 10, 19], E. parvocirrus Nassy and Dupouy, 1988 [ 9, 20], and E. trivolvis (Cort, 1914) [ 9, 21], can be considered to be valid [ 1, 2, 8, 9]. Their differential features, in terms of the life cycle, morphology, and geographical distribution, are reviewed in Table 1. It is of note that the morphology of the adult flukes is almost indistinguishable between E. revolutum, E. jurini, and E. echinatum, with only several minor differences ( Table 1). Kanev et al. [ 18] compared the differential features of the 3 species and proposed that E. jurini be defined for those that have viviparid snails as the first intermediate host, molluscs, frogs, and freshwater turtles as the second intermediate host, and mammals but not birds as the definitive host. By comparison, E. revolutum have lymnaeid snails as the first intermediate host, molluscs, tadpoles, fish, and freshwater turtles as the second intermediate host, and birds but not mammals as the definitive host [ 18]. E. echinatum have lymnaeid and planorbid snails as the first intermediate host, gastropods as the second intermediate host, and both birds and mammals as the definitive hosts [ 18]. Kanev et al. [ 18] also presented differential points in their cercarial morphology, in particular the numbers of penetration gland cell pores and paraesophageal gland cell pores ( Table 1). In our study, the attempt to infect ducks failed and adult flukes were successfully recovered only from hamsters. The second intermediate host was Filopaludina snails, a viviparid molluscan species. The first intermediate host for our flukes may be the same or different species of viviparid snails, although this was not confirmed in our study. These results are compatible with E. jurini rather than E. revolutum. However, this needs to be clarified through molecular and genetic studies, and the taxonomic significance of E. jurini remains to be elucidated. We herein tentatively designated our specimens as E. revolutum.
Another point that needs to be discussed is the size of the metacercariae. Our metacercariae were 170-190 µm in diameter which is larger than those reported for E. jurini (140-160 µm) [ 17] and E. echinatum (120-130 µm) [ 15] (under the name E. lindoensis). With regard to E. revolutum, the metacercarial cyst sizes vary by researchers (117-125 µm by Johnston and Angel [ 22], 132-152 µm by Kanev [ 6], 150-160 µm by Tubangui [ 23], and even 147-220 µm by Zdarska in 1964 as reviewed by Kanev [ 6]). The size of metacercariae may vary depending on the amount of pressure placed on the cover slip. However, it should be further determined whether all metacercariae within these size range belong to a single taxon of E. revolutum. Few studies of echinostome flukes have been performed in Vietnam. Kanev [ 6] studied Cercaria spinifera (presumed to be E. revolutum) in Central Europe and other areas and suggested the possible presence of E. revolutum in Vietnam. Recently, metacercariae of Echinochasmus japonicus were reported from a species of freshwater fish, Labeo rohita in Vietnam [ 24]. In addition, 2 types of echinostome cercariae (species undetermined) were detected from several species of freshwater snails in Nam Dinh Province, Vietnam [ 25]. The Type 2 cercariae [ 25] detected from Gyraulus convexiusculus snails may be those of Echinostoma sp. based on the size and the photo presented. However, this identification needs to be clarified. Regarding the surface topography of E. revolutum adults, there has been surprisingly little documentation. There is one report which focused on the collar region [ 26]. Although several ultrastructural studies were performed on what was thought to be E. revolutum [ 27, 28], the echinostomes studied were later determined to be E. trivolvis [ 26]. The surface ultrastructure of the collar region of E. revolutum appeared to be basically the same in our study and that of Kanev et al. [ 26]. In particular, the end group and lateral group collar spines were almost identical. However, our adult flukes were obtained from experimental hamsters (mammal) whereas those in Kanev et al. [ 26] were harvested from chickens (birds) [ 26]. Therefore, the taxonomic issue needs to be further clarified on our observed worms.
ACKNOWLEDGMENT
This study was supported by the National Foundation for Science and Technology Development (NAFOSTED) in Vietnam.
REFERENCES
1. Chai JY. In Fried B, Toledo R eds, Echinostomes in humans. The Biology of Echinostomes. 2009, New York, USA. Springer. pp 147-183.  2. Fried B, Graczyk TK. Recent advances in the biology of Echinostoma species in the 'revolutum' group. Adv Parasitol 2004;58:139-195. PMID: 15603763.   3. Chai JY, Shin EH, Lee SH, Rim HJ. Foodborne intestinal flukes in Southeast Asia. Korean J Parasitol 2009;47(suppl):S69-S102. PMID: 19885337.    4. Yu SH, Mott KE. Epidemiology and morbidity of food-borne intestinal trematode infections. Trop Dis Bull 1994;91:R125-R152.
5. Sohn WM, Chai JY, Yong TS, Eom KS, Yoon CH, Sinuon M, Socheat D, Lee SH. Echinostoma revolutum infection, Pursat Province, Cambodia. Emerg Infect Dis 2011;17:117-119. PMID: 21192870.  6. Kanev I. Life-cycle, delimitation and redescription of Echinostoma revolutum (Froelich, 1802) (Trematoda: Echinostomatidae). Syst Parasitol 1994;32:61-70.  7. Kanev I, Odening K. Further studies on Cercaria spinifera La Valette, 1855 in Central Europe. Khelmintholgiya 1983;15:24-34.
8. Kostadinova A, Gibson DI. In Fried B, Graczyk TK eds, The systematics of the echinosotmes. Echinostomes as Experimental Models for Biological Research. 2000, Dordrecht, Netherlands. Kluwer Academic Publishers. pp 31-57.  9. Toledo R, Esteban JG, Fried B. Recent advances in the biology of echinostomes. Adv Parasitol 2009;69:147-204. PMID: 19622409.   10. Maldonado A Jr, Vieira GO, Lanfredi RM. Echinostoma luisreyi n. sp. (Platyhelminthes: Digenea) by light and scanning electron microscopy. J Parasitol 2003;89:800-808. PMID: 14533693.   11. Fried B, Huffman J. The biology of the intestinal trematode Echinostoma caproni. Adv Parasitol 1996;38:311-368. PMID: 8701798.   12. Lee SH, Chai JY, Hong ST, Sohn WM. Experimental life history of Echinostoma cinetorchis. Korean J Parasitol 1990;28:39-44.   13. Kechemir N, Jourdane J, Mas-Coma S. Life cycle of a new African echinostome species reproducing by parthenogenesis. J Nat Hist 2002;36:1777-1784.  14. Kostadinova A, Gibson DI, Biserkov V, Chipev N. Re-validation of Echinostoma miyagawai Inshii, 1932 (Digenea: Echinostomatidae) on the basis of the experimental completion of its life cycle. Syst Parasitol 2000;45:81-108. PMID: 10743854.   15. Sandground JH, Bonne C. Echinostoma lindoensis n. sp., a new parasite of man in the Celebes with an account of its life history and epidemiology. Am J Trop Med Hyg 1940;20:511-536.
16. Lie KJ, Basch PF. Life history of Echinostoma barbosai sp. n. (Trematoda: Echinostomatidae). J Parasitol 1966;52:1052-1057. PMID: 5926328.   17. Toledo R, Muňoz-Antoli C, Esteban JG. The life cycle of Echinostoma friedi n. sp. (Trematoda: Echinostomatidae) in Spain and a discussion on the relationships within the ' revolutum' group based on cercarial chaetotaxy. Syst Parasitol 2000;45:199-217. PMID: 10768763.   18. Kanev I, Dimitrov V, Radev V, Fried B. Redescription of Echinostoma jurini (Skvortzov, 1924) with a discussion of its identity and characteristics. Ann Naturhist Mus Wien 1995;97B:37-53.
19. Lie KJ, Basch PF. The life history of Echinostoma paraensei sp. n. (Trematoda: Echinostomatidae). J Parasitol 1967;53:1192-1199. PMID: 6078607.   20. Nassi H, Dupouy J. Experimental study of the life history of Echinostoma parvocirrus n. sp. (Trematoda: Echinostomatidae), a larval parasite of Biomphalaria glabrata in Guadeloupe. Ann Parasitol Hum Comp 1988;63:103-118 (in French).
21. Kanev I, Fried B, Dimitrov V, Radev V. Redescription of Echinostoma trivolvis (Cort, 1914) (Trematoda; Echinostomatidae) with a discussion of its identity. Syst Parasitol 1995;32:61-70.  22. Johnston TH, Angel LM. The life history of Echinostoma revolutum in South Australia. Trans Roy Soc South Austral 1941;65:317-322.
23. Tubangui MA. Observations on the life histories of Euparyphium murinum Tubangui, 1931 and Echinostoma revolutum (Froelich, 1802) (Trematoda). Phil J Sci 1932;47:497-513.
24. Chi TT, Dalsgaard A, Turnbull JF, Tuan PA, Murrell KD. Prevalence of zoonotic trematodes in fish from a Vietnamese fish-farming community. J Parasitol 2010;94:423-428. PMID: 18564743.  25. Dung BT, Madsen H, The DT. Distribution of freshwater snails in family-based VAC ponds and associated waterbodies with special reference to intermediate hosts of fish-borne zoonotic trematodes in Nam Dinh Province, Vietnam. Acta Trop 2010;116:15-23. PMID: 20457118.   26. Kanev I, Dezfuli BS, Nestorov M, Fried B. Scanning electron microscopy of the collar region of Deropristis inflata and Echinostoma revolutum. J Helminthol 1999;73:51-57.
27. Smales LR, Blankespoor KD. Echinostoma revolutum (Troelich, 1802) Looss, 1899 and Isthmiophora melis (Schrank, 1788) Lűhe, 1909 (Echinostomatidae, Digenea): Scanning electron microscopy of the tegumental surfaces. J Helminthol 1984;58:187-195.  28. Fried B, Fujino T. Scanning electron microscopy of Echinostoma revolutum (Trematoda) during development in the chick embryo and the domestic chick. Int J Parasitol 1984;14:75-81. PMID: 6706466.  
Fig. 1
Filopaludina sp. snails purchased from a local market in Nam Dinh Province, Vietnam. 
Fig. 2
Metacercaria (170 µm in diameter) of E. revolutum from a Filopaludina sp. snail, Nam Dinh, Vietnam. 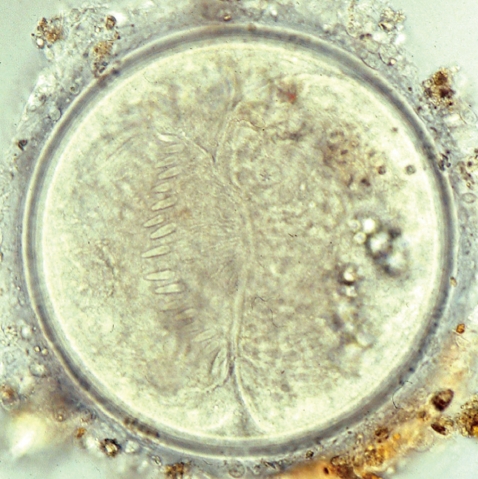
Fig. 3
Adult worm (7.0×1.1 mm) of E. revolutum experimentally obtained from a hamster at day 14 post-infection. 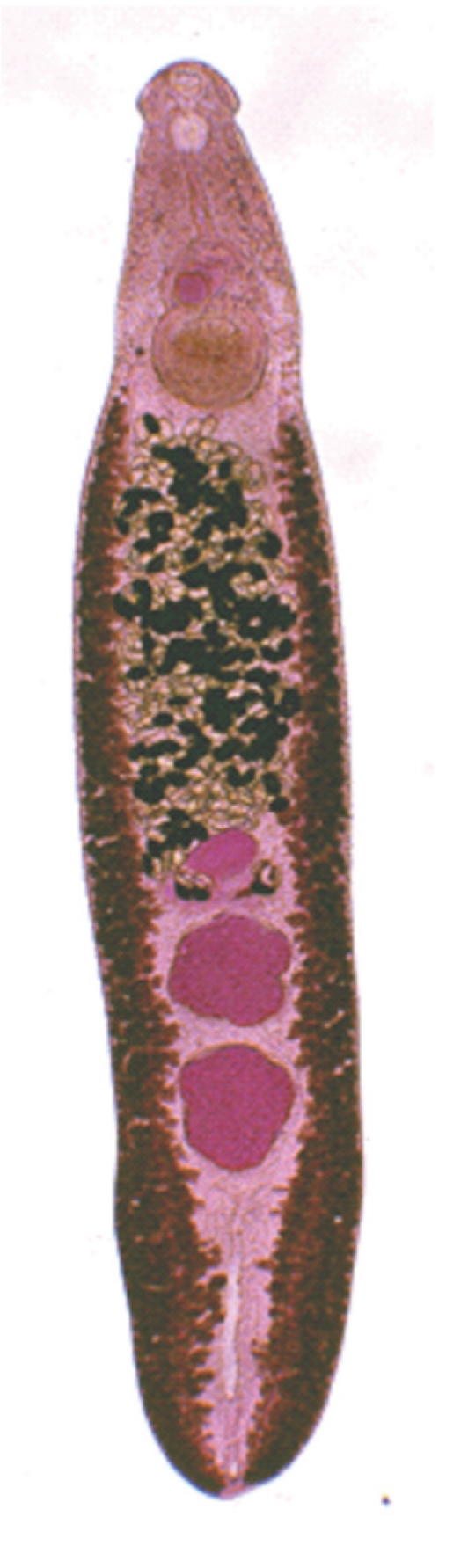
Fig. 4
Egg (114×72 µm in size) of E. revolutum in the feces of an infected hamster. 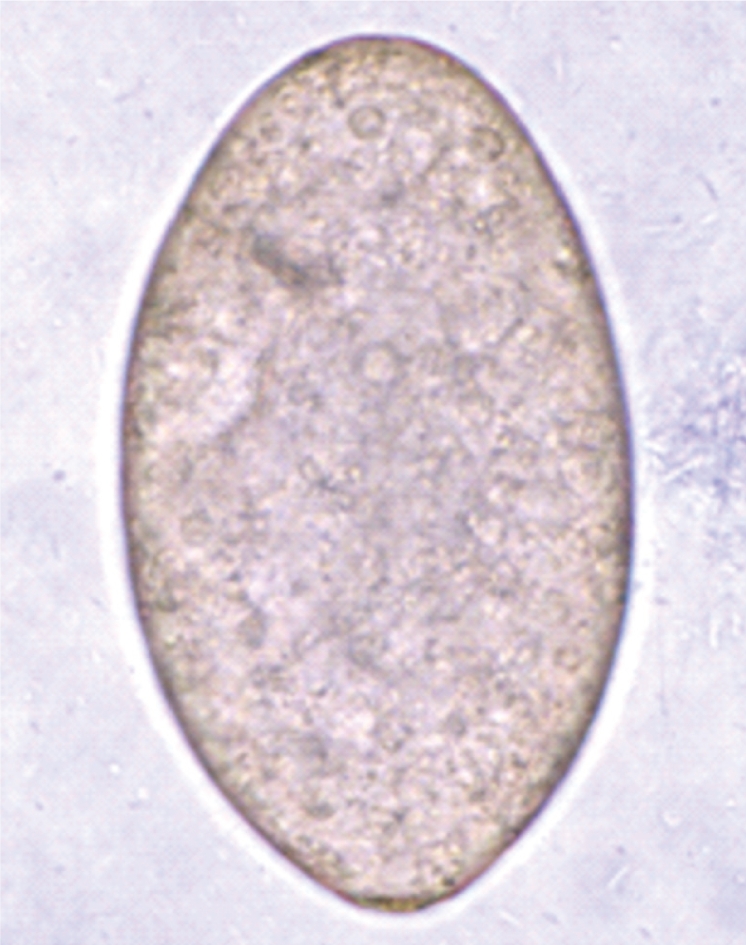
Fig. 5
Scanning electron microscopic (SEM) views of E. revolutum adults. Ventral view of a whole worm showing its elongated and leaf-like appearance, with a distinct head collar, and oral and ventral suckers. 
Fig. 6
Scanning electron microscopic (SEM) views of E. revolutum adults. The tegument around the oral sucker and head collar showing its smooth surface and numerous sensory papillae and the characteristic arrangement of the collar spines, including the 5 corner spines. The tegument posterior to the head collar is covered with scale-like spines. 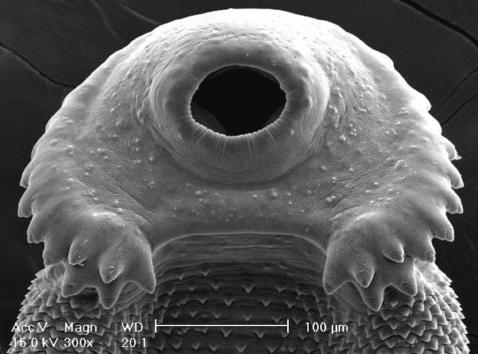
Fig. 7
Scanning electron microscopic (SEM) views of E. revolutum adults. The lip of the ventral sucker covered by many sensory papillae. An unarmed cirrus is seen protruded from the cirrus sac. 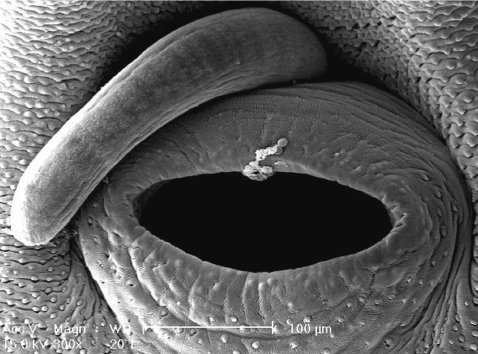
Table 1.
Characteristic features of the 37-collar-spined Echinostoma belonging to the ‘revolutum’ group
|
Species |
Adult worm morphology & egg size |
Larval morphology (size) |
1st intermediate host (snails) |
2nd intermediate host (freshwater animals) |
Definitive hosts |
Geographic distributiona
|
Reference |
|
E. caproni (syn. E. liei, E. togoensis) |
Two testes confluent/adjacent |
Cercaria (body): 328×144 μm |
Biomphalaria
|
Gastropods |
Birds (chick, duck, pigeon) |
Africa (Madagascar) |
[9-11] |
|
Body broadest at just posterior to acetabulum |
Metacercarial cyst: 150 μm (diam.) |
Tadpoles |
|
Bulinus
|
Mammals (mouse, rat, hamster) |
|
A ttenuated posterior end Eggs: 117×75 μm |
|
E. cinetorchis
|
A bnormal location/disappearance of testes |
Cercaria (body): 163×109 μm |
Hippeutis Segmentina
|
Tadpoles |
Mammals (including man, rat, mouse, dog) |
Asia (Korea, Japan, China) |
[1,12] |
|
Metacercarial cyst: 139 μm (diam.) |
Fish |
|
Eggs: 99-116 x 65-76 μm |
Gastropods |
|
E. deserticum
|
Parthenogenetic reproduction |
Cercaria (body): 306×133 μm |
Bulinus
|
Gastropods |
Birds |
Africa (Niger, Algeria) |
[9,13] |
|
Presence of 0, 1, 2 testes |
Metacercarial cyst: 146 μm (diam.) |
Mammals |
|
Eggs: 58-74×36-46 μm |
|
E. echinatum(syn. E. lindoense, E. barbosai, E. robustum) |
Deeply lobed testes |
Cercaria (body): 300-460×140-180 μm |
Anisus
|
Gastropods |
Birds |
Europe |
[14-16] |
|
Cirrus sac never extending to middle of acetabulum |
Biomphalaria
|
Mammals (including man) |
Asia (Celebes) |
|
No. of penetration gland pores: 6 |
South America (Brazil) |
|
Eggs: 92-124×65-76 μm |
No. of paraesophageal gland pores: 60-64 |
|
Metacercarial cyst: 120-130 μm (diam.) |
|
E. friedi
|
Cirrus sac extending to anterior margin of acetabulum |
Cercaria (body): 223-327×118-185 μm |
Lymnaea,
|
Gastropods |
Bird |
Europe (Spain) |
[9,17] |
|
Radix
|
Mammals (mouse) |
|
No. of penetration gland pores: 6 |
Gyraulus, Biomphalaria
|
|
Testes usually lobated |
No. of paraesophageal gland pores: 46-50 |
|
Eggs: 83-117×54-76 μm |
|
Metacercarial cyst: 131-173 μm (diam.) |
Bulinus
|
|
E. jurini (syn. E. bol-schewense) |
Cirrus sac extending to middle portion of acetabulum |
Cercaria (body): 327-445×159-254 μm |
Viviparus
|
Gastropods |
Mammals |
Europe (Bulgaria, Czech Republic, Slovakia) Asia |
[9,18] |
|
Molluscs |
|
No. of penetration gland pores: 6 |
Frogs |
|
Testes smooth or slightly irregular in outline |
No. of paraesophageal gland pores: 8-10 |
|
Eggs: 96-132×72-88 μm |
Metacercarial cyst: 140-160 μm (diam.) |
|
E. luisreyi
|
Smaller oral lateral corner spines and larger aboral lateral corner spines |
Cercaria (body): 417×181 μm |
Physa
|
Gastropods |
Mammals (mouse, hamster) |
South America (Brazil) |
[9,10] |
|
Metacercarial cyst: 171 μm (diam.) |
|
Dorsally situated excretory pore |
|
Eggs: 89-113×65-82 μm |
|
E. miyagawai
|
Very elongate body |
Cercaria (body): 312-340×146-203 μm |
Planorbis
|
Gastropods |
Birds |
Europe (Bulgaria, Czech Republic) |
[9,14] |
|
Large head collar |
Planorbarius
|
Mammals |
|
Small ventral sucker |
No. of penetration gland pores: 6 |
|
Testes indented and subglobular |
No. of paraesophageal gland pores: 42-46 |
Anisus
|
|
Lymnaea
|
Asia (Japan, Korea) |
|
Long cirrus sac extending to middle portion of cetabulum |
Metacercarial cyst: 144-154 μm (diam.) |
|
Eggs: 95×60 μm |
|
E. paraensei
|
5-11 of dorsalmost collar spines smaller than others |
Cercaria (body): 228-275×117-136 μm |
Biomphalaria
|
Gastropods |
Mammals (hamster, mouse, rat) |
South America (Brazil) |
[9,10,19] |
|
Eggs: 104-122×74-86 μm |
No. of penetration gland pores: 6-8 |
Physa
|
|
Paraesophageal glands absent |
|
Metacercarial cyst: 132-148 μm (diam.) |
|
E. parvocirrus
|
Small cirrus |
Cercaria (body): 300-410×120-150 μm |
Biomphalaria
|
Gastropods |
Birds |
West Indies (Guadeloupe) |
[9,20] |
|
Eggs: 101-115×55-70 μm |
|
No. of penetration gland pores: 6 |
|
No. of paraesophageal gland pores: 24 |
|
Metacercarial cyst: 130-150 μm (diam.) |
|
E. revolutum(syn. E. audyi, E. paraulum, E. ivaniosi) |
Cirrus sac extending to middle of acetabulum |
Cercaria (body): 265-315×128-154 μm |
Lymnaea
|
Gastropods |
Birds |
Europe |
[1,6,9] |
|
Bivalves |
Mammals (including man) |
Asia |
|
Testes smooth or slightly lobated |
No. of penetration gland pores: 4 |
Fish |
North America |
|
No. of paraesophageal gland pores: 16-20 |
Tadpoles |
|
Eggs: 88-113×61-74 μm |
Turtles |
|
Metacercarial cyst: 132-152 μm (diam.) |
|
|
E. trivolvis (syn. E. rodriguesi) |
Body slightly plump |
Cercaria (body): 300-450×150-250 μm |
Helisoma
|
Gastropods |
Birds (pigeon, duck, chicken) |
North America |
[9,21] |
|
Cirrus sac extending to middle of acetabulum |
Biomphalaria
|
Mussels |
South America (Brazil) |
|
No. of penetration gland pores: 6 |
Planarians |
Mammals (hamster, mouse, rat) |
|
Testes smooth, oval, or slightly irregular in outline |
No. of paraesophageal gland pores: 4-6 |
Tadpoles |
|
Fish |
|
Eggs: 90-130×60-70 μm |
Metacercarial cyst: 135-170 μm (diam.) |
Amphibians |
|
Turtles |
|
|













