AbstractLung fluke, Paragonimus heterotremus, is a flatworm causing pulmonary paragonimiasis in cats, dogs, and humans in Southeast Asia. We examined the ultrastructure of the testis of adult P. heterotremus with special attention to spermatogenesis and spermiogenesis using scanning and transmission electron microscopy. The full sequence of spermatogenesis and spermiogenesis, from the capsular basal lamina to the luminal surface, was demonstrated. The sequence comprises spermatogonia, spermatocytes with obvious nuclear synaptonemal complexes, spermatids, and eventual spermatozoa. Moreover, full steps of spermatid differentiation were shown which consisted of 1) early stage, 2) differentiation stage representing the flagella, intercentriolar body, basal body, striated rootlets, and electron dense nucleus of thread-like lamellar configuration, and 3) growing spermatid flagella. Detailed ultrastructure of 2 different types of spermatozoa was also shown in this study.
INTRODUCTIONParagonimiasis is an important food-born parasitic zoonosis endemic in many parts of Asia, Africa, and South America [1]. There are about 50 species, of which 11 are known to cause infections in humans [2]. In Southeast Asia, Paragonimus heterotremus has been increasingly detected as an important cause of infection in humans [3]. The parasite utilizes 2 intermediate hosts and completes the life cycle in wild mammals such as tigers, civet cats, toddy cats, dogs, mongooses, and humans [4]. Freshwater molluscan species serve as the first intermediate host and freshwater crab species serve as the common second intermediate host [2]. Worms generally mature in pairs in capsules in the lungs of their mammalian hosts [4-6].
Spermatogenesis and spermiogenesis have been subjects often used for phylogenetic study of the parasitic Platyhelminthes [7-14]. Spermiogenesis presents minor differences in Digenea [8,15-18], but the full range of variation in the taxon has not yet been explored. Although light and electron microscopic features of the spermatogenesis of some Paragonimus species, such as Paragonimus miyazakii, Paragonimus westermani and Paragonimus ohirai have already been examined [19-22], those of P. heterotremus, remain unstudied. This study presents the ultrastructure of the spermatogenesis and spermiogenesis in P. heterotremus.
MATERIALS AND METHODSMetacercariae of P. heterotremus were collected from freshwater crabs, Larnaudia larnaudii, at Chet Khot waterfall, Saraburi Province, Thailand. Adult worms were collected from the lungs of a cat which had been infected with metacercariae 2-4 months earlier. The worms were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB), pH 7.2, overnight. The fixed specimens were divided into 2 groups, and they were further processed for transmission electron microscopy (TEM) or scanning electron microscopy (SEM).
For TEM, the specimens were post-fixed in 1% osmium tetroxide in 0.1 M PB at pH 7.2 for 1 hr, dehydrated with a graded series of ethanol concentrations, infiltrated with propylene oxide for 20 min and embedded in epoxy epon 812. Semithin sections were cut from the embedded specimen blocks using an ultramicrotome (Leica EM UC6) and were stained with 1% toluidine blue solution for light microscopic examination to identify the morphological features representing the full sequence of differentiation, i.e., stages of spermatogenesis and spermiogenesis. Thereafter, ultrathin sections were cut, and after double-staining with uranyl acetate and lead citrate observed and photographed using a Hitachi-H600 (Hitachi, Tokyo, Japan) transmission electron microscope.
For SEM, fixed specimens were dissected under a binocular microscope and testes were extirpated and subsequently fixed with 1% osmium tetroxide fixative for 1 hr and dehydrated with a graded series of ethanol concentrations. Thereafter, the dehydrated specimens were dried with a critical point dryer (Hitachi HCP-2, Tokyo, Japan) and mounted on cupper stubs with silver paste and observed under SEM (Hitachi-SU3400, Tokyo, Japan).
RESULTSTEMBased on light microscopic findings representing the full sequence of spermatogenesis and spermiogenesis from the capsule to the luminal surface (Fig. 1), ultrastructural features were carefully examined. In individual primary spermatogonia, comparatively large oval nuclei with prominent nucleoli in euchromatin were tightly packed and laid on the folded basement membrane beneath the testicular capsule. Several mitochondria and fine electron-dense ribosomes were present in scanty cytoplasm (Fig. 2A, B). In much more advanced spermatogonia, patches of heterochromatin were noted in the nucleus, and the cytoplasm was slightly more voluminous containing cytoplasmic organelles, especially mitochondria and ribosomes (Fig. 2C, D). Eight primary spermatocytes, in accordance with established understanding that these came from 3 mitotic divisions of an individual spermatogonium, formed a group with a rosette-like appearance, gradually developing a central common mass called a cytophore (Fig. 3A, B). In early spermatocytes, pale round nuclei were larger than those of spermatogonia, and each had an obvious nucleolus (Fig. 3C). The synaptonemal complexes were also demonstrated in the nuclei of early prophase-I meiotic division of the primary spermatocytes (Fig. 3D, E). At late prophase-I meiotic division, an irregularly shaped nucleus without a nucleolus was noted, in which the chromatin was condensed into chromosomes (Fig. 3F, G).
Meiosis-I is known to produce 16 secondary spermatocytes. During this stage, the volume of the cytophore gradually increased. The nuclei of secondary spermatocytes were much more electron-dense, 4-fold smaller than those of primary spermatocytes and with an increased nucleo-cytoplasmic ratio (Fig. 4A). The nuclei were dislocated away from the central cytophore which showed completely fused cell membranes with the neighbors. The proximal plasma membranes between neighboring spermatocytes became highly convoluted (Fig. 4B). It was followed by meiosis-II which produces 32 spermatids from 16 spermatocytes. The earliest spermatids were of elongated oval shape, and the cell membranes at their apical margins were associated with electron-dense undercoats (Fig. 5A, B). Their nuclei were oval in shape and had chromatin of fine thread-like reticular arrangement intermingled with nucleoli. At the stage of spermiogenesis, in which the spermatids become differentiated into the spermatozoa, the spermatid nuclei were increasingly elongated and curved in shape. The nuclear chromatin changed into bundles of electron dense lamellar scroll-like configurations which were arranged in parallel with the longitudinal axis of the nuclei, resulting in a honeycomb-like appearance in cross section (Fig. 5C-E). A cone-shaped projection of the cytoplasm appeared on the surface of the cytophore in conjunction with each nucleus. It contained several kinds of cytoplasmic organelles such as mitochondria, Golgi apparatus, rough endoplasmic reticulum and free ribosomes (Fig. 6A). As the spermatid-cone grew outward, its base sank slightly into the cytoplasm of the cytophore, forming a shallow ring-like groove delineated by arching membranes of increasing electron density. The thickening membranes close to the cytophore, called zone of differentiation, showed a density increase associated with the arching membranes, which was found to be a single row of microtubules immediately underneath the cell membrane (Fig. 6A). The median cytoplasmic process (MCP) arising away in an opposite direction from the cytophore was also shown (Fig. 6A). The flagellar complex also arose from the zone of differentiation, and was composed of a middle piece of the intercentriolar body sandwiched by 2 flagellar axonemes which anchored the basal bodies and the striated rootlets, respectively (Fig. 6B-D).
The flagellar axonemes consisted of 9 doublet tubules concentrically arranged around a single central core complex (Fig. 6C, D). At the early stage of flagellar development, they were arranged almost perpendicular to the MCP axis (Fig. 6B-D). As the development proceeded, the emergent flagella were rotated and arranged closer and in parallel to the longitudinal axis of the MCP of the developing spermatozoon (Fig. 7A-C) and they finally fused with the MCP. The arching membrane portions also gradually moved centrally to make a narrow constricted portion (Fig. 7A-D) and then finally pinched off the head portion of the spermatid from the central mass of the cytophore, resulting in formation of the spermatozoon (Fig. 7E). In synchrony with this change, mitochondria grew in the long axis of the spermatid and spermatozoon. Subsequently large vacuoles appeared in the cytophore, which represents a stage of degeneration (Fig. 7F). Spermatozoa, when pinched off, did not contain the central body, centriole and striated rootlets anymore. However, axonemes still remained and they were already fused with the MCP (Fig. 8). Two different types of spermatozoa were distinguished; the first type with translucent cytoplasm and the second type with densely packed glycogen granules (Fig. 8). Rows of microtubules were arranged underneath the cell membrane of the head and middle portions of spermatozoa of both types. Additionally, the elongated mitochondria also appeared centrally in the core of both types. Elongated nuclei of high electron density were also apparent in both types.
SEMThe SEM observations were focused on the spermatids and spermatozoa. Flagella of fine thread-like appearance were shown to extend from the cone-shaped proximal region of the spermatids (Fig. 9A). The pinched-off head and thicker elongated tails of the spermatozoa were also shown (Fig. 9C). Spermatids and mature spermatozoa were shown to be characterized by frequent occurrence of flagella and tails bundles respectively (Fig. 9).
DISCUSSIONThe ultrastructural organization of the spermatogenesis and spermiogenesis of P. heterotremus are in accord with those previously reported for other digeneans [12-17]. An exception to this general pattern is noticed: Two ultrastructurally distinct types of spermatozoa differentiated in P. heterotremus; one with electron-translucent cytoplasm, which is commonly found in other Digenea, and the other with highly packed glycogen granules which has not been reported in other digeneans except Dicrocoelium hospes and Hypocreadium caputvadum [12,23]. The finding of this exception contrasts sharply with some previous previous reports of spermatozoa ultrastructure of other digeneans [8,15,18-22].
The synaptonemal complexes were readily observed in the primary spermatocytes of P. heterotremus as previously noted in other digeneans which suggested the showing of normal chromosome paring [8,11,21]. Since the secondary spermatocytes are quite few in number, when compared with the other stages of development, probably due to the fact that this stage occurs shortly before dividing into the spermatids, it remains unknown whether or not the synaptonemal complexes occur in the secondary spermatocytes.
The development of the zone of differentiation during spermiogenesis is followed by the formation of 2 free flagella. Initially, the flagella are freely arranged in a direction perpendicular to the spermatid longitudinal axis, and they can bend and finally fuse with the MCP. Such fusion has also been observed in Aspidogastrea, Monogenea, Gyrocotylidae, Amphilinidea, Eucestoda, and Haploporidae within the Digenea [7]. The presence of 2 flagella, 2 centrioles with striated rootlets, and this fusion are generally characteristics of Platyhelminthes [11-13]. This study has not observed the numerous flagella projecting from the 1 cell as previously observed in P. westermani [21] which may represent difference in their parthenogenesis.
The elongation of the nucleus along the longitudinal axis of the spermatid and the condensation of the chromatin into lamellae have already been observed in other digeneans [15,18, 19,21]. When features of nuclei and chromatins are compared between P. heterotremus and other species of Digenea at differentiation stages from the spermatogonia to the spermatozoa, the spermatogonia of P. heterotremus have oval nuclei similar to those of Saccacocoelioides [18], but different from those of P. ohirai [16] and P. pulmonalis [21] which have round-shaped nuclei and from those of Fasciola hepatica which has irregular nuclei [24]. The patchy heterochromatin of the spermatogonia was consistent with that of the others except for F. hepatica which contains quite large amounts of heterochromatin [24]. The large, round, and euchromatic nuclei of primary spermatocytes of P. heterotremus are similar to those of P. westermani [21] but are different from those of F. hepatica which have oval nuclei with dense heterochromatin [24]. The nuclei of spermatids during several steps of differentiation into the spermatozoa are similar to those of Paragonimus spermatids reported previously [19-22].
ACKNOWLEDGMENTSThis study was supported by the Electron Microscopy Unit, Department of Anatomy, Faculty of Medicine, Khon Kaen University; Research and Diagnostic Center for Emerging Infectious Diseases, Khon Kaen University; the Higher Education Research Promotion National Research University Project of Thailand, Office of the Higher Education Commission through the Health Cluster (SHep-GMS), Thailand; and the TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004. The authors gratefully thank visiting Prof. Hisatake Kondo for proof reading of the manuscript.
REFERENCES1. Procop GW. North American paragonimiasis (caused by Paragonimus kellicotti) in the context of global paragonimiais. Clin Microbiol Rev 2009;22:415-446. PMID: 19597007.
2. Miyazaki I. Lung flukes in the world: morphology and life history. Symposium on epidemiology of parasitic disease. Tokyo, Japan. International Medical Foundation of Japan. 1974, pp 101-134.
3. Doanh PN, Le NT, Tat D. Paragonimus and paragonimiasis in Vietnam. Arizono N, Chai JY, Nawa Y, Takahashi Y. Asian Parasitology Vol. 1. Food-borne helminthiasis in Asia. Chiba, Japan. Federation of Asian Parasitologists. 2005, pp 149-153.
4. Singh TS, Sugiyama H, Rangsiruji A. Paragonimus and paragonimiasis in India: review article. Indian J Med Res 2012;136:192-204. PMID: 22960885.
5. Vélez I, Velásquez LE, Vélez ID. Morphological description and life cycle of Paragonimus sp. (Trematoda: Troglotrematidae): causal agent of human paragonimiasis in Columbia. J Parasitol 2003;89:749-755. PMID: 14533686.
6. Guan XH. Paragonimiasis. In Li YL, Guan XH eds, Human Parasitology. Beijing, China. People's Public Health Press. 2005, pp 109-113.
7. Justine JL. Phylogeny of parasitic Platyhelminthes: a critical study of synapomorphies proposed on the basis of the ultrastructure of spermiogenesis and spermatozoa. Can J Zool 1991;69:1421-1440.
8. Rohde K, Watson NA, Cribb T. Ultrastructure of sperm and spermatogenesis of Lobatostoma manteri (Trematoda, Aspidogastrea). Int J Parasitol 1991;21:409-419. PMID: 1917282.
9. Justine JL. Spermatozoal ultrastructure and phylogeny in the parasitic Platyhelminthes. Mèm Mus Natn Hist Nat 1995;166:55-86.
10. Hathaway MA, Hathaway RP, Kritsky DC. Spermatogenesis in Octomacrum lanceatum (Monogenoidea, Oligonchoinea, Mazocraeidea). Int J Parasitol 1995;25:913-922. PMID: 8550291.
11. Diniz Baptista-Farias MF, Kohn A, Cohen SC. Spermatogenesis and spermiogenesis in Microcotyle sp. (Microcotylidae, Monogenea). J Parasitol 1999;85:832-838. PMID: 10577717.
12. Agostini S, Miquel J, Ndiaye PI, Marchand B. Dicrocoelium hospes Looss, 1907 (Digenea, Dicrocoeliidae): spermiogenesis, mature spermatozoon and ultrastructural comparative study. Parasitol Res 2005;96:38-48. PMID: 15772868.
13. Seck MT, Marchand B, Bâ CT. Ultrastructure of spermiogenesis and the spermatozoon of Paramphistomum microbothrium [Fischoeder 1901] (Digenea: Paramphistomidae), a parasite of Bos taurus in Senegal. Parasitol Res 2007;101:653-662. PMID: 17401579.
14. Quilichini Y, Foata J, Marchand B. Ultrastructural study of the spermatozoon of Pronoprymna ventricosa (Digenea, Baccigerinae), parasite of the twaite shad Alosa fallax Lacepede (Pisces, Teleostei). Parasitol Res 2007;101:1125-1130. PMID: 17594115.
15. Hendow HT, James BL. Ultrastructure of spermatozoon and spermatogenesis in Maritrema linguilla (Digenea: Microphallidae). Int J Parasitol 1988;18:53-63. PMID: 3366537.
16. Orido Y, Hata H. Cytological and cytofluorometrical studies on gametogenesis of Paragonimus ohirai (Trematoda: Troglotrematidae). Int J Parasitol 1988;18:95-101.
17. Watson NA, Rohde K. Re-examination of spermatogenesis of Multicotyle purvisi (Platyhelminthes, Aspidogastrea). Int J Parasitol 1995;25:579-586. PMID: 7635635.
18. Baptista-Farias MFD, Kohn A, Cohen SC. Ultrastructure of spermatogenesis and sperm development in Saccocoelioides godoyi Kohn & Froes, 1986 (Digenea, Haploporidae). Mem Inst Oswaldo Cruz 2001;96:61-70. PMID: 11285476.
19. Sato M, Oh M, Sakoda K. Electron microscopic study of spermatogenesis in the lung fluke (Paragonimus miyazakii). Z Zellforsch Mikrosk Anat 1967;77:232-243. PMID: 5627215.
20. Hirai H, Tada I. Morphological features of spermatozoa of Paragonimus ohirai (Trematoda: Platyhelminthes) examined by a silver nitrate staining technique. Parasitology 1991;103:103-110. PMID: 1945518.
21. Fujino T, Ishii Y. Ultrastructural studies on spermatogenesis in a parthenogenetic type of Paragonimus westermani (Kerbert 1878) proposed as P. pulmonalis (Baelz 1880). J Parasitol 1982;68:433-441. PMID: 7097441.
22. Orido Y. Ultrastructure of spermatozoa of the lung fluke, Paragonimus ohirai (Trematoda: Troglotrematidae), in the seminal receptacle. J Morphol 1988;196:333-343. PMID: 3418719.
23. Kacem H, Bakhoum AJ, Eira C, Neifar L, Miquel J. Ultrastructural characters of the spermatozoon of the digenean Hypocreadium caputvadum Kacem et al., 2011 (Lepocreadioidea: Lepocreadiidae), an intestinal parasite of Balistes capriscus in Tunisia. C R Biol 2012;335:637-644. PMID: 23199631.
24. Stitt AW, Fairweather I. Spermatogenesis and the fine structure of the mature spermatozoon of the liver fluke, Fasciola hepatica (Trematoda: Digenea). Parasitology 1990;101:395-407. PMID: 2092295.
Fig. 1Thick section with toluidine staining photograph of the adult P. heterotremus testis showing stages of spermatogenesis and spermiogenesis. *Differentiated spermatids. C, capsule; PS, primary spermatocyte; SG, spermatogonia; SS, secondary spermatocyte. 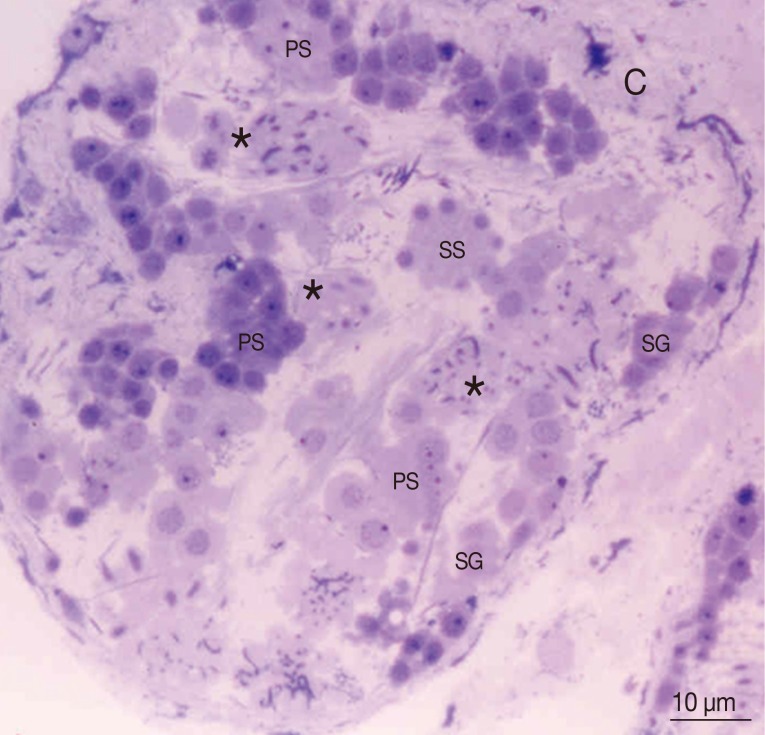
Fig. 2Transmission electron micrographs of a testis in adult P. heterotremus showing the spermatogonia. (A, B) Spermatogonia laid on the folding capsular basement membrane (BM) beneath the testicular capsule (C). (C, D) Advanced spermatogonia with patches of heterochromatin (arrowhead) in the nucleus (N) and slightly more voluminous cytoplasm containing cytoplasmic organelles. *Euchromatin, Arrow: ribosome. Ce, centriole; M, mitochondria. 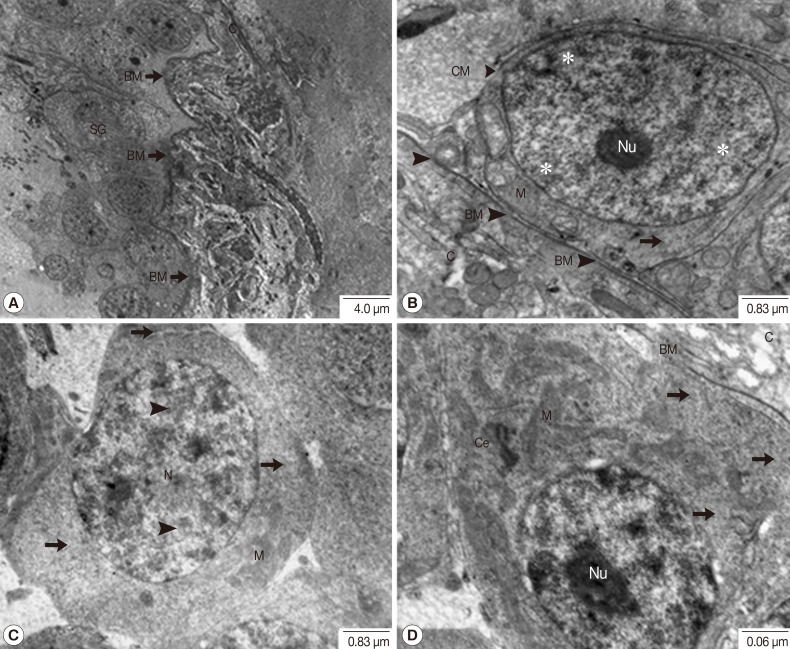
Fig. 3Transmission electron micrographs of an adult P. heterotremus testis showing full stages of primary spermatocyte differentiation and meiotic-I division. (A, B) Primary spermatocytes (PS) form a group with a rosette-like appearance connected at cytophore (CP). (C-E) Large euchromatic nucleus (N) of early meiosis-I of the primary spermatocyte with obvious nucleolus (Nu) and synaptonemal complexes (arrowhead) with intact nuclear membrane. (F, G) Primary spermatocyte at the late prophase-I meiotic division with irregular shaped nuclei in which the chromatins were condensed to the chromosomes (Ch), and the nuclear membrane gradually disappeared (arrowhead). Thin arrow: ribosome; Thick arrow: cell membrane. M, mitochondria. 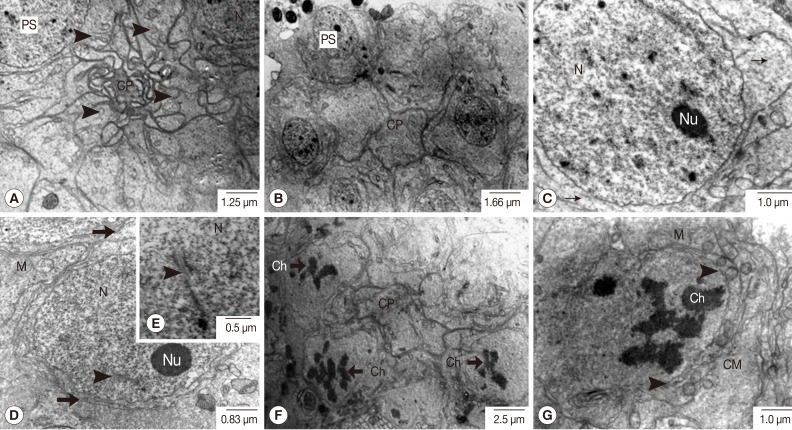
Fig. 4Transmission electron micrographs of an adult P. heterotremus testis showing the secondary spermatocytes. (A) Oval nuclei (N) of secondary spermatocytes were much more electron-dense with the increased nucleo-cytolasmic ratio. (B) Nuclei were dislocated away from the central cytophore (CP), and proximal plasma membranes between neighboring spermatocytes became highly convoluted (arrow). 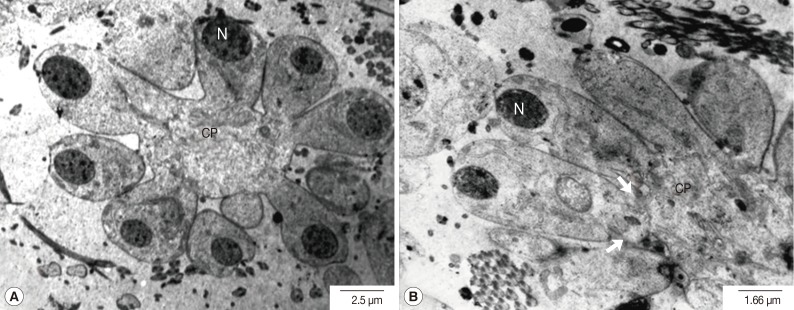
Fig. 5Transmission electron micrographs of an adult P. heterotremus testis showing the early stage of differentiation of spermatids. (A, B) The earliest spermatids were elongated and oval-shaped (arrowhead) with oval nuclei (N) and intermingled nucleoli (Nu). The cell membranes at their apical margin were associated with electron-dense undercoats (arrow). (C-E) The elongated and curved spermatid nuclei had bundles of electron-dense lamellar nuclear chromatins arranged in parallel with the longitudinal axis of nuclei (N), and the nuclei took honeycomb-like appearance in cross section (arrowhead). Arrow: ribosome. CM, cell membrane; Gol, Golgi apparatus; M, mitochondria; RER, rough endoplasmic reticulum. 
Fig. 6Transmission electron micrographs of an adult P. heterotremus testis showing the flagellar complex. (A) Median cytoplasmic process (MCP), cone-shaped projection of the cytoplasm appeared on the surface of the cytophore in conjunction with individual nucleus (N) with zone of differentiation (ZD) and arching membrane (arrowhead). (B-D) The flagellar complexes (Fg), arranged almost perpendicular to the MCP axis, were composed of a middle piece of the intercentriolar body (Ib), 2 flagellar axonemes (AX) which anchored the basal bodies (BB), and the striated rootlets (Sr). Arrow: microtubules. M, mitochondria; N, nucleus; RER, rough endoplasmic reticulum. 
Fig. 7Transmission electron micrographs of an adult P. heterotremus testis showing the spermiogenesis. (A-D) Flagella (Fg) were rotated and arranged closer and in parallel to the longitudinal axis of the median cytoplasmic process (MCP) and nuclei (N) of the spermatids. They finally fused with the MCP. The arching membrane (Ar) also gradually moved centrally to make a narrow constricted portion. (E, F) The head portion of the spermatozoon pinched off from the cytophore (CP). Large vacuoles appeared (arrowhead) in the cytophore. BB, basal body; M, mitochondria; Sr, striated rootlets; SZ, spermatozoa; ZD, zone of differentiation. 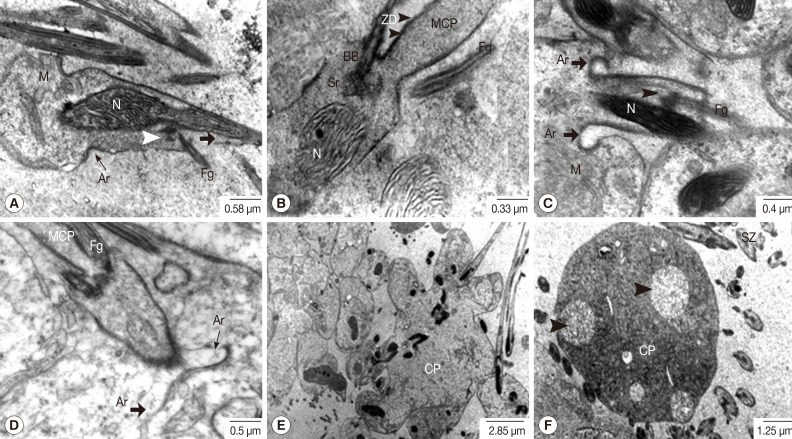
Fig. 8Transmission electron micrographs of an adult P. heterotremus testis showing 2 types of the spermatozoa which represent head, middle, and tail portions. (A, B) Translucent type. (C-E) Densely packed glycogen granular type. Arrowhead: cell membrane. AX, axoneme; H, head portion; M, mitochondria; Mid, middle portion; N, nucleus; T, tail portion. 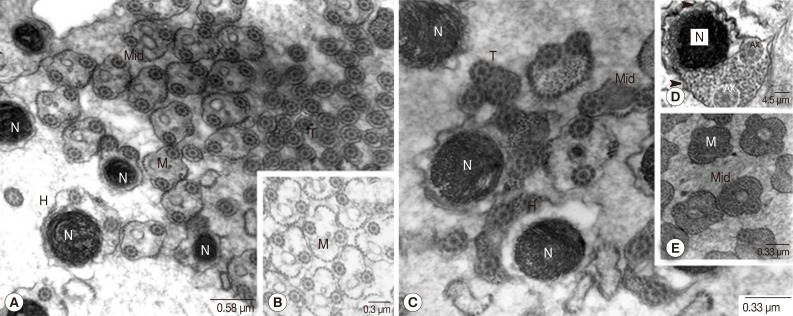
Fig. 9Scanning electron micrographs of an adult P. heterotremus testis showing spermatids with flagella and spermatozoa with tails. (A, B) Cone-shaped spermatids (ST) with rectangle thin flagella (thin white arrow) and the bundle of flagella (Fg). (C) The pear-shaped spermatozoon (arrow) with its thicker central outgrowth tail (arrowhead) wandering inside the luminal surface of the testis above the group of spermatocytes (SC). (D) A bundle of the tails of spermatozoa (arrowhead) intermingled with some spermatid flagella (Fg). 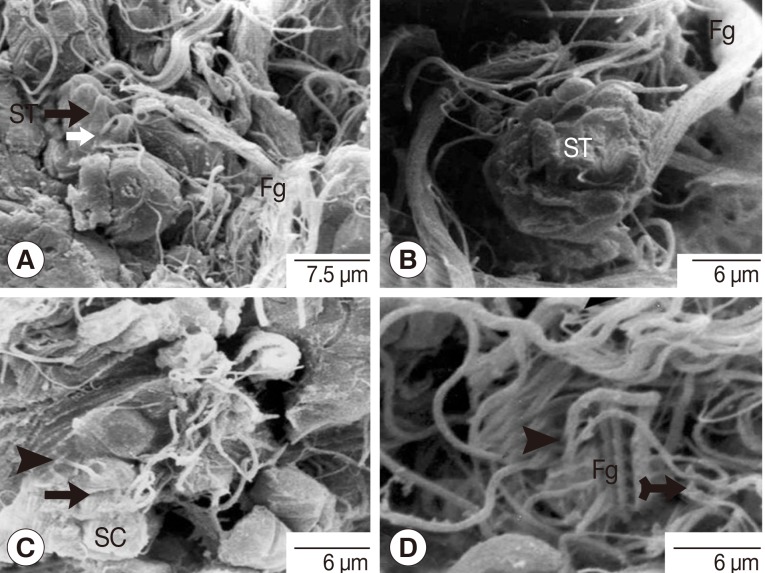
|
|
|||||||||||||||||||||||||||||||||||||||||||