Santos and Borges: Current Knowledge of Small Flukes (Digenea: Heterophyidae) from South America
Abstract
Fish-borne heterophyid trematodes are known to have a zoonotic potential, since at least 30 species are able to infect humans worldwide, with a global infection of around 7 million people. In this paper, a ‘state-of-the-art’ review of the South American heterophyid species is provided, including classical and molecular taxonomy, parasite ecology, host-parasite interaction studies and a list of species and their hosts. There is still a lack of information on human infections in South America with undetected or unreported infections probably due to the information shortage and little attention by physicians to these small intestinal flukes. Molecular tools for specific diagnoses of South American heterophyid species are still to be defined. Additional new sequences of Pygidiopsis macrostomum, Ascocotyle pindoramensis and Ascocotyle longa from Brazil are also provided.
Key words: Ascocotyle longa, review, trematodosis, fish parasite, checklist
INTRODUCTION
The Opisthorchioidea Looss, 1899 (Digenea) comprises a group of species of medical and veterinary importance with a worldwide distribution for which approximately 100 life cycles have been described [ 1– 6]. Heterophyids are usually parasitic as adults in mammals and birds, and utilize mollusks and then generally fishes as intermediate hosts. Such fish-borne heterophyids are known to have a zoonotic potential. According to Chai and Jung [ 7], 30 species are able to infect humans worldwide, with a global level of infection of around 7 million people. Integrative studies, including molecular data associated with structural, morphological and ecological aspects, have helped elucidate the taxonomy and phylogeny of the group [ 6], although, based on limited molecular evidence, Thaenkham et al. [ 8, 9] have questioned the recognition of this family as distinct from the Opisthorchiidae. It is clear that further taxonomic studies of the Heterophyidae are required, since recognition is the basis for prevention and control programs for these emerging fish-borne trematodoses [ 10]. Not only taxonomic problems but also other dubious aspects of the biology of these parasites need to be solved via the use of molecular tools [ 11]. According to Chai and Lee [ 12], of the approximately 70 species of intestinal trematodes that parasitize humans, more than 30 belong to Heterophyidae. Humans acquire these fish-borne zoonotic trematode (FZT) infections by eating raw or inadequately cooked fish. The main symptoms include abdominal pain, flatulence, diarrhea, eosinophilia, lethargy and anorexia. Occasional severe symptoms or even deaths have been observed when heterophyid eggs reach the blood or lymphatic vessels and migrate to the cardiac muscle and brain [ 1, 12]. In this paper we present a review of the research on South American (SA) heterophyid trematodes.
TAXONOMY AND LIFE CYCLES
The genus Ascocotyle Looss, 1899 is represented by numerous species composing the so called “ Ascocotyle complex” [ 13, 14]. The distribution of those species in different genera and subgenera represented a problem in the taxonomy of the group. The most commonly used classification was based on Sogandares-Bernal and Lumsden [ 13] which considered Ascocotyle subdivided into 3 sub-genera: Ascocotyle ( Ascocotyle), Ascocotyle ( Leighia), and Ascocotyle ( Phagicola). The subgenera Ascocotyle and Leighlia included adults with 2 rows of oral spines and vitellarium extending up to the ventral sucker; the former had the uterus confined posterior to ventral sucker and a parapleurolofocercous-type cercaria while in the latter the uterus extended to the pharynx level and had ophthalmogymnocephalous-type cercaria. The subgenus Phagicola comprised adults with 1, 2 or no rows of oral spines, vittelarium extending only to the ovary level and the cercaria was of pleurolofocercous type [ 13]. Pearson [ 15] revised the family Heterophyidae and did not recognize subfamilies or subgenera, considering the following genera occurring in SA: Acanthotrema Travassos, 1928, Ascocotyle Looss, 1899 (syn. Phagicola Faust, 1920; Parascocotyle Stunkard & Haviland, 1924; Metascocotyle Ciurea, 1933; Pseudascocotyle Sogandares-Bernal & Bridgman, 1960; Leighia Sogandares-Bernal & Lumsden, 1963); Centrocestus Looss, 1899, Cryptocotyle Lühe, 1899, Heterophyes Cobbold, 1886, Opisthometra Poche, 1926, Pholeter Odhner, 1914, and Pygidiopsis Looss, 1907 (syn. Caiguiria Nasir & Diaz, 1971). Several authors continued using the subgenera of Ascocotyle [ 16– 18]. The WoRMS database [ 19] followed Pearson’s taxonomy [ 15] and authors tend to avoid using subgenera for the stability of the taxa. The current list of species occurring in SA with their hosts and geographical distribution is presented in Table 1. The host names are based on ITIS online database [ 20]. Species of the genus Ascocotyle are the most prevalent in SA and include Ascocotyle angeloi Travassos, 1928 (syn. A. rara), Ascocotyle angrense Travassos, 1916, Ascocotyle cameliae Hernández-Orts, Georgieva, Landete & Scholz, 2019, Ascocotyle diminuta (Stunkard & Haviland, 1924), Ascocotyle felippei Travassos, 1928, Ascocotyle hadra Ostrowski de Núñez, 1992, Ascocotyle longa Ransom, 1920, Ascocotyle patagoniensis Hernández-Orts, Montero, Crespo, García, Raga & Aznar, 2012, Ascocotyle pindoramensis (Travassos, 1928), Ascocotyle secunda Ostrowski de Núñez, 2001 and Ascocotyle tertia Ostrowski de Núñez, 2001.
Out of these, a species considered as causing an emerging fish-borne disease in humans is A. longa, a parasite recorded throughout the Americas, Europe, Africa and the Middle East [ 1, 21– 25]. Its life-cycle includes adult parasites in the intestine of fish-eating birds and mammals and the metacercaria mainly in mullets ( Mugil spp.) [ 14, 18, 24, 26– 31]. The life cycle of A. longa is associated with estuaries and coastal lagoons, where the mugilid juveniles become infected [ 7, 17, 24, 26– 28, 30]. The first intermediate host in Argentina, Brazil and Uruguay is the snail Heleobia australis (d’Orbigny, 1835), in which rediae and parapleurolophocercous cercariae develop [ 7, 31]. Metacercariae have been found encysted in the body musculature, heart, stomach, liver, kidney, spleen, gonads and mesentery of the mullets [ 17, 26, 27, 32]. Galván-Borja et al. [ 18] examined mullets from Colombia and reported that these metacercariae cause inflammatory reactions involving macrophage aggregates and necrosis, thus suggesting that trematodes infecting the liver of mugilids can affect the growth and well-being of the fish. The life-cycles of A. diminuta, A. angrense, A. hadra, A. felippei, A. secunda and A. tertia were studied in Argentina and follow similar pattern to that of A. longa and the adults mainly parasitize fish-eating birds and mammals [ 6, 33– 36] ( Table 1). Three species of Pygidiopsis have been reported in SA: Pygidiopsis macrostomum Travassos, 1928, Pygidiopsis crassus Ostrowski de Núñez, 1995 and Pygidiopsis australis Ostrowski de Núñez, 1996. Pygidiopsis macrostomum was originally described based on a single specimen obtained from the intestine of Rattus norvegicus (Erxleben, 1777) in Brazil [ 37], but was subsequently reported from the intestine of the bulldog-bat Noctilio leporinus mastivus Ribeiro, 1914 in Cuba [ 38, 39]. This parasite was redescribed using morphological, ultrastructural and molecular data [ 8, 40], and its life cycle was completed using naturally infected snails and fish, and hamsters as experimental definitive hosts [ 4]. The snail H. australis acts as the first intermediate host, the fishes Phalloptychus januarius (Hensel, 1868), Jenynsia multidentata (Jenyns, 1842) and Poecilia vivipara (Bloch & Schneider, 1801) as second intermediate hosts, and fish-eating mammals and birds as definitive hosts [ 4]. Indirect immunocytochemistry and phalloidin-fluorescence techniques, allied with confocal laser scanning microscopy, were also used by Borges et al. [ 8] to describe the muscular and neuronal structures of Pygidiopsis macrostomum, revealing the complex arrangement of muscular fibers and ganglia within the body. Pygidiopsis crassus was reported from Argentina and Venezuela while Pygidiopsis australis only from Argentina; metacercariae were found in small fish, and adults in Himantopus melanurus (Viellot, 1817) and experimentally infected Gallus gallus (Linnaeus, 1758) [ 5, 6, 41, 42].
Centrocestus formosanus Nishigori, 1924, a species considered of zoonotic importance, was introduced in the Americas within its host snail Melanoides tuberculata. It has been reported from Brazil [ 43– 47], Colombia [ 48], Peru [ 50] and Venezuela [ 51]. The life-cycle includes naturally infected fish (metacercariae in the gills) and birds (adults in the intestine) and experimental infections were performed with G. gallus [ 45] ( Table 1). To our knowledge there are no reports on human infections by C. formosanus in SA.
Cryptocotyle thapari McIntosh, 1953 described from the otter Pteronura brasiliensis Zimmermann, 1780 from U.S. National Zoo in Washington, D.C. was later redescribed from specimens collected from Lontra longicaudis (Olfers, 1818) (= Lutra longicaudis) from Bolivia [ 52, 53]. Cryptocotyle dominicana Casalins, Arbetman, Viozzi & Altman, 2020 was described from Larus dominicanus Lichtenstein, 1823, and the metacercariae from Galaxias platei Steindachner, 1898 were conspecific based on morphometrics and genetic markers [ 48].
Few records of Galactosomum spp. have been reported from Brazil, Colombia and Peru from marine birds [ 54– 58]. Opistometra planicollis (Rudolphi, 1819) was only recorded from the same bird Sula leucogaster (Boddaert, 1783) in both countries [ 59, 60]. Some species have been only reported from Venezuela, such as Haplorchis pumilio (Looss, 1896), Pholeter anterouterus Fischtal & Nasir, 1974 and Stictodora sp. Pholeter anterouterus and Stictodora sp. were reported parasitizing birds while H. pumilio considered an introduced species parasitizes the introduced M. tuberculata, the fish Anablepsoides harti and the bird Butorides striatus, and experimentally infected ducks [ 50, 54, 61– 63] ( Table 1).
MOLECULAR STUDIES
Sequences of the 18S, 28S, and ITS2 rDNA regions of Pygidiopsis macrostomum and mtDNA cox-1 sequences of Ascocotyle pindoramensis have already been described [ 8]. Molecular phylogeny linked P. macrostomum and A. pindoramensis with other heterophyids but, in agreement with Thaenkham et al. [ 10], the distinction of the Heterophyidae and Opisthorchiidae remained unclear. Additionally, new sequences of P. macrostomum, A. pindoramensis and A. longa from the nuclear regions 18S, 28S, and ITS2 rDNA and the mitochondrial marker mtDNA cox-1 are hereby presented and deposited in GenBank ( Table 2), following the methodology of Borges et al. [ 8]. The sequence analysis of the 18S and 28S rDNA regions of these species did not exhibit any intraspecific variability, but some variability was observed between the species. BLAST analyses indicated a high similarity with other heterophyiid genera and species deposited in GenBank. Concatenated phylogenetic analyses (18S and 28S) readily differentiated the heterophyid genera Metagonimus, Haplorchis and Procevorum ( Fig. 1), which demonstrates that these gene regions can be useful for solving low-level taxonomic problems when using concatenated genes with higher evolutionary rates. Martorelli et al. [ 17], Dzikowski et al. [ 64] and Alda et al. [ 9] sequenced the 18S rDNA region of A. longa from Israel and Argentina, the sequences of which are deposited in GenBank under the accession numbers AY245703, JX093559, and KF697717, respectively. The comparison of the genetic variability of 18S rDNA among these and our sequences of A. longa (MF980220 and MF980222) resulted in high values of the p distance ranging from 1.6 to 3.3%. This indicates the necessity of a wider, more comprehensive study of A. longa, comparing samples from different geographical regions in order to verify the existence of a complex of sibling species. The comparison of ITS2 rDNA sequences of P. macrostomum resulted in 3 haplotypes with an intraspecific p distance ranging from 0.4% to 1.9% between them. Such a level of variability appears to be acceptable according to Miller and Cribb [ 65] who reported 5% variability among species of heterophyids. However, a comparison of new sequences of the ITS2 rDNA of A. longa and A. pindoramensis did not result in any intraspecific variation. Similar results with heterophyids of the genus Haplorchis were reported [ 66] without intraspecific variability using the ITS2 rDNA region. The intrageneric distance, based on sequences of the ITS2 rDNA of the species A. longa and A. pindoramensis, was as high as 12.8%, indicating that the ITS2 region is a good marker for identifying closely related species. According to Brusentsov et al. [ 67], genetic variability provides species survival and resilience to ecosystems. Phylogenetic analysis carried out using the mtDNA cox-1 region resulted in poorly supported branches, which may indicate a lack of phylogenetic signal of this marker for comparing species from higher taxa, such as at the family level ( Fig. 2). Vanhove et al. [ 68] and Besansky et al. [ 69] discussed the problematic use of generic primers for highly variable regions, such as the mtDNA cox-1, for elucidating taxonomic relationships between platyhelminth families, proposing the use of 28S rDNA and ITS rDNA regions as more reliable barcodes for this phylum.
BEHAVIOR AND ECOTOXICOLOGY
The influence of the metacercariae of A. pindoramensis on the behavior of the fish-host Poecilia vivipara was investigated using an image system linked to a video camera able to record the locomotory activity of the fishes before and after experimental infections [ 70]. The results indicated a significant decrease in the swimming behavior of fish after 14 days post-infection when metacercariae were fully developed, correlated with parasite intensity [ 70]. The heterophyid trematode C. formosanus was introduced into Brazil with the gastropod Melanoides tuberculata, which has a wide geographic distribution in the Neotropics [ 47]. This species, which is considered to have zoonotic potencial, is able to alter the locomotory activity of its snail host regardless of the standard length [ 47]. Ecotoxicological and behavioral experiments with heterophyids were performed in order to study the effect of cyanobacteria on both the parasite (metacercaria) and its fish host. The fish Poecilia vivipara is a common host of Pygidiopsis macrostomum off the coast of Rio de Janeiro. This fish-trematode interaction was tested as a model for ecotoxicological studies with the cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) [ 71, 72]. Changes in the motility of metacercariae of P. macrostomum occurred after their fish host was exposed to different concentrations of crude lyophilized extract of the cyanobacterium CYRF-01 producer of neurotoxic saxitoxins (STX), resulting in a temporary paralysis [ 72]. Interestingly, their motility recovered after the fish were kept for 48 hr in clean water, suggesting that blooms of cyanobacteria may interfere with the motility of both the fish host and its parasites.
TREMATODOSIS
Ascocotyle longa has a wide geographic distribution, comprising the Mediterranean Sea and the coasts of the North Atlantic, South Atlantic and Pacific [ 73]. In SA, it has been reported parasitizing different mugilid fishes from Venezuela, Peru and Brazil [ 73– 76]. This species has also been reported as parasitizing avian hosts [ 77, 78] and dogs in the USA, Chile, Brazil, and Peru [ 79– 84].
In Brazil, only few cases of human trematodosis caused by heterophyids have been reported; these were from São Paulo [ 85– 87]. However, records of metacercariae in mullets from off the Brazilian coast are common [ 24, 26, 75, 76, 88– 90]. Following a report of the occurrence of metacercariae of A. longa with a prevalence of 100% in mullets from the Rodrigo de Freitas Lagoon, Rio de Janeiro, an alert was given concerning the possibility of a related human disease [ 24, 26]. The spleen was reported to be the most parasitized organ in these fishes, with 100% of prevalence, followed by the heart, intestinal wall, liver and muscles, with prevalence decreasing from 98 to 87%. In other organs, such as the stomach wall, brain, gonads and gall bladder, the prevalence decreased from 70 to 30% [ 26]. Associated experimental infections using only small pieces of muscle tissue to fed hamsters resulted in a higher intensity of infection during spring/summer than in autumn/winter. However, the potential risk of infection was considered to be high in view of the high prevalence and intensity of A. longa in the muscles of mullets throughout the year [ 26]. Gueretz et al. [ 91] reported the prevalence of A. longa of 87% in Mugil curema and 100% in Mugil liza off Santa Catarina, southeastern Brazil, confirming the high prevalence on mugilid fish. Similar prevalence indices have been found in mugilids from Colombia and Uruguay [ 18, 73]. Although heterophyid species are already included in the List of Risk Classification of Biological Agents by the Brazilian Health Ministry [ 92], human cases are no longer reported. Studies on the viability of heterophyid metacercariae of A. longa have been perfomed [ 93, 94] with methodological standardization performed by Borges et al. [ 95]. Metacercariae isolated from M. liza and incubated in mullet muscle tissue at different temperatures showed that all metacercariae were dead after heating for 15 min at 60˚C, 100˚C and 180˚C. When frozen, all metacercariae died after 2 hr of exposure to −35˚C and −20˚C, but 24 hr of exposure to −10˚C was necessary to kill all metacercariae in the fillets [ 95]. The specific procedures for inactivation of parasites was important to prevent outbreaks of trematodiases caused by A. longa. Regarding the species with zoonotic potential such as C. formosanus, H. pumilio and Cryptocotyle spp., human cases have not been so far reported.
CONCLUSIONS
Our current knowledge of heterophyid species occurring in SA has enabled us to have a comprehensive view of the species distribution, their hosts and studies on taxonomy and life cycles, along with their influence on their host and the environment in terms of behavioral studies and ecotoxicologic interactions with cyanobacteria.
Asian species of heterophyids are constantly under study in relation to human and animal infections, but, for SA species, these data are still scarce. In this region, information concerning the effects of these trematodes on host tissues is needed, as are new diagnostic approaches for a better understanding of the epidemiology of this trematodoses and its geographic distribution. Marked definida por portesclaudia
The identification of the emerging risk of heterophyid species as a human health hazard relating to the ingestion of raw fish represents a preventive instrument at the disposal of authorities, and molecular approaches under study will provide new insights for the development of diagnostic tools.
AVAILABILITY OF DATA AND MATERIALS
The DNA sequences generated as a part of this study were deposited to the GenBank ( Table 2).
ACKNOWLEDGMENT
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (001), PAEF/FIOCRUZ and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Universal n. 449658/2014-7).
Conflict of interest
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
REFERENCES
2. Toledo R, Esteban JG, Fried B. Immunology and pathology of intestinal trematodes in their definitive hosts. Adv Parasitol 2006;63:285-365.  3. Simões SBE, das Neves RFC, Santos CP. Life history of Acanthocollaritrema umbilicatum Travassos, Freitas and Bührnheim, 1965 (Digenea: Cryptogonimidae). Parasitol Res 2008;103:523-528.    4. Simões SB, Barbosa HS, Santos CP. The life history of Pygidiopsis macrostomum Travassos, 1928 (Digenea: Heterophyidae). Mem Inst Oswaldo Cruz 2009;104:106-111.    5. Ostrowski de Núñez M. Life history of Pygidiopsis crassus n. sp. (Trematoda, Digenea, Heterophyidae) in the Neotropical region. Mem Inst Oswaldo Cruz 1995;90:13-19.    6. Ostrowski de Núñez M. Life cycles of two new sibling species of Ascocotyle (Ascocotyle) (Digenea, Heterophyidae) in the Neotropical Region. Acta Parasitol 2001;46:119-129.
7. Chai JY, Jung BK. Fishborne zoonotic heterophyid infections: an update. Food Waterborne Parasitol 2017;8–9:33-63.  8. Borges JN, Costa VS, Mantovani C, Barros E, Santos EGN, Mafra CL, Santos CP. Molecular characterization and confocal laser scanning microscopic study of Pygidiopsis macrostomum (Trematoda: Heterophyidae) parasites of guppies Poecilia vivipara
. J Fish Dis 2017;40:191-203.   9. Alda P, Bonel N, Panei CJ, Cazzaniga NJ, Martorelli SR. First molecular identification of Ascocotyle ( Phagicola) longa in its first intermediate host the mud snail Heleobia australis
. Acta Parasitol 2015;60:791-795.    10. Thaekham U, Blair D, Nawa Y, Waikagul J. Families Opisthorchiidae and Heterophyidae: are they distinct? Parasitol Int 2012;61:90-93.   11. Skov J, Kania PW, Dalsgaard A, Jørgensen TR, Buchmann K. Life cycle stages of heterophyid trematodes in Vietnamese freshwater fishes traced by molecular and morphometric methods. Vet Parasitol 2008;160:66-75.   12. Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int 2002;51:129-154.   13. Soganders-Bernal F, Lumsden RD. The generic status of the heterophyid trematodes of the Ascocotyle complex, including notes on the systematics and biology of Ascocotyle angrense Travassos, 1916. J Parasitol 1963;49:264-274.  14. Scholz T. Taxonomic study of Ascocotyle ( Phagicola) longa Ransom, 1920 (Digenea: Heterophyidae) and related taxa. Syst Parasitol 1999;43:147-158.   15. Pearson J. Family Heterophyidae Leiper, 1909. In Bray RA, Gibson DI, Jones A eds, Keys to the Trematoda. 3:Wallingford, UK. CAB International. 2008, pp 113-141.  16. Hernández-Orts JS, Georgieva S, Landete DN, Scholz T. Heterophyid trematodes (Digenea) from penguins: a new species of Ascocotyle Looss, 1899, first description of metacercaria of Ascocotyle ( A.) patagoniensis Hernández-Orts, Montero, Crespo, García, Raga and Aznar, 2012, and first molecular data. Int J Parasitol Parasites Wildl 2019;8:94-105.    17. Martorelli SR, Lino A, Marcotegui P, Montes MM, Alda P, Panei CJ. Morphological and molecular identification of the fish-borne metacercaria of Ascocotyle ( Phagicola) longa Ransom, 1920 in Mugil liza from Argentina. Vet Par 2012;190:599-603.  18. Galván-Borja D, Olivero-Verbel J, Barrios-García L. Occurrence of Ascocotyle ( Phagicola) longa Ransom, 1920 (Digenea: Heterophyidae) in Mugil incilis from Cartagena Bay, Colombia. Vet Parasitol 2010;168:31-35.   19. WoRMS Editorial Board. World Register of Marine Species [Internet]; [cited 2020 May 21]. Available from: http://www.marinespecies.org at VLIZ.. 2020. 20. Integrated Taxonomic Information System (ITIS). On-line database [Internet]; Available from: https://www.itis.gov/
21. Muller R, Wakelin D. Worms and Human Diseases. 2nd ed. Wallingford, UK. CAB International. 2002, p 300.
22. Chai JY. Human Intestinal Flukes: from Discovery to Treatment and Control. Dordebrecht, The Netherlands. Springer. 2019, pp 1-167.
23. Scholz T, Aguirre-Macedo ML, Salgado-Maldonado G. Trematodes of the family Heterophyidae (Digenea) in Mexico a review of species and new host and geographical records. J Nat Hist 2001;35:1733-1772.  24. Simões SB, Barbosa H, Santos CP. The life cycle of Ascocotyle ( Phagicola) longa (Digenea: Heterophyidae), a causative agent of fish-borne trematodosis. Acta Trop 2010;113:226-233.   26. Santos CP, Lopes KC, Costa VS, Santos EGN. Fish-borne trematodosis: potential risk of infection by Ascocotyle ( Phagicola) longa (Heterophyidae). Vet Parasitol 2013;193:302-306.   27. Montes MM, Marcotegui PS, Martorelli SR. Digeneos parásitos de juveniles de Mugil liza (Pisces: Mugilidae) en la Bahía de Samborombón, Argentina, con el reporte de metacercarias zoonóticas de Ascocotyle (Phagicola) longa
. Rev Arg Parasitol 2013;1:97-124 (in Spanish)..
28. Montes MM, Martorelli SR. An ecological and comparative analysis of parasites in juvenile Mugil liza (Pisces, Mugilidae) from two sites in Samborombón bay, Argentina. Iheringia Ser Zool 2015;105:403-410 (in Spanish)..   29. Carnevia D, Speranza G. Seasonal variations in parasites found in mullet (Mugil platanus Günther, 1880) juveniles captured on the Uruguayan coast of the River Plate. Bull Eur Ass Fish Pathol 2003;23:245-249.
30. Fernandez JB. Los parásitos de la lisa Mugil cephalus L., en Chile: sistematica y aspectos poblacionales (Perciformes: Mugilidae). Gayana Zool 1987;51:3-58.
31. Carnevia D, Perretta A, Venzal JM, Castro O.
Heleobia australis (Mollusca, Hydrobiidae) y Mugil platanus (Pisces, Mugilidae), primer y segundo hospedador intermediario de Ascocotyle (Phagicola) longa (Digenea, Heterophyidae) en Uruguay. Rev Bras Parasit Vet 2004;13:283 (in Spanish)..
32. Jara CA, Escalante H.
Phagicola arnaldoi: identificacion de sus metacercarias obtenidas de Mugil cephalus
. Hidrobios 1982;6:37-43.
33. Ostrowski de Núñez M. Life-history studies of heterophyid trematodes in the Neotropical Region: Ascocotyle ( Phagicola) diminuta (Stunkard & Haviland, 1924) and A. ( P.) angrense Travassos, 1916. Syst Parasitol 1993;24:191-199.   34. Ostrowski de Núñez M. Life cycle of Ascocotyle (Phagicola) angeloi (Digenea: Heterophyidae) in the Neotropical Region. Folia Parasitol 1998;45:199-204.
35. Ostrowski de Núñez M. Life history studies of heterophyid trematodes in the Neotropical region: Ascocotyle ( Leighia) hadra sp.n. Mem Inst Oswaldo Cruz 1992;87:539-543.   36. Ostrowski de Núñez M. Fauna de agua dulce en la República Argentina. IV. Las cercarias de Ascocotyle (A.) tenuicolis Price 1935 y de Pygidiopsis pindoramensis Travassos 1929 (Trematoda, Heterophyidae). Physis Sec B 1976;35:51-57.
37. Travassos L. Deux nouvelles espèces du genre Ascocotyle Looss, 1899. Comptes Rendues 1928;100:939-940.
38. Odening K. 1969;Exkretionssystem und systematische Stellung kubanischer Fledermaustrematoden. Bijdr Dierk 39:45-62.  39. Zdzitowiecki K, Rutkowska MA. The helminthofauna of bats (Chiroptera) from Cuba. III. A review of trematodes. Acta Parasitol Polonica 1980;26:201-214.
40. Simões SBE, Barbosa HS, Santos CP. Redescription and surface ultrastructure of Pygidiopsis macrostomum (Digenea: Heterophyidae). J Parasitol 2005;91:931-936.   41. Ostrowski de Núñez M. Life history studies of heterophyid trematodes in the Neotropical Region: Pygidiopsis australis sp. n., a sibling species of P. pindoramensis Travassos, 1929. Acta Parasitol 1996;41:13-19.
42. Alda P, Martorelli SR, Sarria R. Digenean parasites in the white-backed stilt Himantopus melanurus vieillot, 1817 (Recurvirostridae) from the Argentine Coast. Comp Parasitol 2011;78:217-219.  43. Pinto HA, de Melo AL.
Melanoides tuberculata (Mollusca: Thiaridae) as an intermediate host of Centrocestus formosanus (Trematoda: Heterophyidae) in Brazil. Rev Inst Med Trop Sao Paulo 2010;52:207-210.    44. Pinto HA, Melo AL. Metacercariae of Centrocestus formosanus (Trematoda: Heterophyidae) in Australoheros facetus (Pisces: Cichlidae) in Brazil. Rev Bras Parasitol Vet 2012;21:334-337.    45. Pinto HA, Gonçalves NQ, López-Hernandez D, Pulido-Murillo EA, Melo AL. The life cycle of a zoonotic parasite reassessed: Experimental infection of Melanoides tuberculata (Mollusca: Thiaridae) with Centrocestus formosanus (Trematoda: Heterophyidae). PLoS ONE 2018;13:e0194161.    46. Bogéa T, Cordeiro FM, Gouveia JS.
Melanoides tuberculatus (Gastropoda: Thiaridae) as intermediate host of Heterophyidae (Trematoda: Digenea) in Rio de Janeiro metropolitan area, Brazil. Rev Inst Med Trop São Paulo 2005;47:87-90.   47. Santos EGN, Costa VS, Santos CP. Does the trematode Centrocestus formosanus affect the locomotory activity of the mollusc Melanoides tuberculatus? Parasit Vectors 2013;6:92-96.     48. Casalins LM, Arbetman MP, Viozzi GP, Flores VR. A new species of Cryptocotyle (Digenea: Heterophyidae) infecting kelp gull and a galaxiid fish in Patagonian freshwater environments: morphological and molecular analyses. J Parasitol 2020;106:203-210.   49. Velásquez LE, Bedoya JC, Areiza A, Vélez I. First record of Centrocestus formosanus (Digenea: Heterophyidae) in Colombia. Rev Mex Biodivers 2006;77:119-121.
50. Pullido-Murillo EA, Furtado LFV, Melo AL, Rabelo EML, Pinto HA. Fishborne zoonotic trematodes transmitted by Melanoides tuberculata snails, Peru. Emerg Infec Dis 2018;24:606-608.  51. Díaz MT, Hernández LE, González G. Studies on the life history of Centrocestus formosanus Nishigori, 1924 (Trematoda: Heterophyidae) in Venezuela. Parasitol Int 1998;47:300.  52. McIntosh A. 1953, A new heterophyid trematode from a Brazilian otter. Thapar Commemoration Volume. University of Lucknow. Lucknow, India. pp 209-210.
53. Gardner SL, Thew PT. Redescription of Cryptocotyle thapari McIntosh, 1953 (Trematoda: Heterophyidae), in the river otter Lutra longicaudis from Bolivia. Comp Parasitol 2006;73:20-23.  54. Ostrowski de Núñez M. Fishes as definitive or intermediate hosts of Opisthorchioid trematodes in South America. Wiad Parazytol 1999;45:329-336.  55. Kreiter A, Semenas L. Helmintos parasitos de Larus dominicanus en la Patagonia Argentina. Bol Chil Parasitol 1997;52:39-42 (in Spanish)..  56. Tantaleán M, Sarmiento L, Huiza A. Digeneos (Trematoda) del Peru. Bol Lima 1992;80:47-84.
57. Travassos L. Contribuição ao conhecimento dos Heterophyidae observados no Brasil. Thése Vet 1929;1-31.
58. Travassos L, Freitas JFT, Kohn A. Trematódeos do Brasil. Mem Inst Oswaldo Cruz 1969;67:1-886.  59. Thatcher VE. Trematódeos Neotropicais. Manaus Brazil. Instituto Nacional de Pesquisas da Amazonia. 1993, pp 1-553.
60. Vélez I. Some digested trematodes of Sula dactylatra (birds) in northern Colombia. Actual Biol 1980;9:3-11.
61. Fischtal JH, Nasir P. Some digenetic trematodes from freshwater and marine fishes of Venezuela. Norweg J Zool 1974;22:71-80.
62. Lutz A. Estudios sobre trematodes observados en Venezuela. Estúdios de Zoologia y Parasitologia Venezoelanas 1928;101-125.
63. Díaz MT, Hernandez LE, Bashirullah AT. Studies on the life cycle of Haplorchis pumilio (Looss, 1896) (Trematoda: Heterophyidae) in Venezuela. Rev Cient 2008;18:35-42.
65. Miller TL, Cribb TH. Two new cryptogonimid genera Beluesca n. gen. and Chelediadema n. gen. (Digenea: Cryptogonimidae) from tropical Indo-West Pacific Haemulidae (Perciformes). Zootaxa 2007;1543:45-60.  66. Thaenkham U, Dekumyoy P, Komalamisra C, Sato M, Dung DT, Waikagul J. Systematics of the subfamily Haplorchiinae (Trematoda: Heterphyidae), based on nuclear ribosomal DNA genes and ITS2 region. Parasitol Int 2010;59:460-465.   67. Brusentsov II, Katokhin AV, Brusentsova IV, Shekhovtsov SV, Borovikov SN, Goncharenko GG, Lider LA, Romashov BV, Rusinek OT, Shibitov SK, Suleymanov MM, Yevtushenko AV, Mordvinov VA. Low genetic diversity in wide-spread Eurasian liver fluke Opisthorchis felineus suggests special demographic history of this trematode species. PLoS One 2013;8:e62453.    68. Vanhove MP, Tessens B, Schoelinck C, Jondelius U, Littlewood DT, Artois T, Huyse T. Problematic barcoding in flatworms: a case-study on monogeneans and rhabdocoels (Platyhelminthes). ZooKeys 2013;365:355-379.  69. Besansky NJ, Severson DW, Ferding MT. DNA barcoding of parasites and invertebrate disease vectors: what you don’t know can hurt you. Trends Parasitol 2003;19:545-546.   70. Santos EGN, Santos CP. Parasite-induced and parasite development-dependent alteration of the swimming behavior of fish hosts. Acta Trop 2013;127:56-62.  71. Lopes KC, Ferrão Filho AS, Santos EGN, Cunha RA, Santos CP. Effects of crude extracts of a saxitoxin-producer strain of the cyanobacterium Cylindrospermopsis raciborskii on the swimming behavior of wild and laboratory reared guppy Poecilia vivipara
. Toxicon 2017;129:44-51.   72. Lopes KC, Ferrão-Filho AS, Santos EGN, Santos CP. First report of neurotoxic effect of the cyanobacterium Cylindrospermopsis raciborskii on the motility of trematode metacercariae. J Helminthol 2018;92:244-249.   73. Carnevia D, Castro O, Perretta A, Venzal JM. Identificación en Uruguay de metacercarias de Ascocotyle (Phagicola) longa Digenea: Heterophyidae parasitando lisas, Mugil platanus Pisces: Mugilidae y evaluación del riesgo de zoonosis y afecciones en mascotas. Veterinaria (Montevideo) 2005;40:19-23.
74. Armas de Conroy G. Investigaciones sobre la fagicolosis en lisas (Mugilidae) de aguas americanas. I. Estudios taxonómicos de Phagicola sp. (Trematoda: Heterophyidae) en mugílidos sudamericanos. Rev Ibér Parasitol 1986;46:39-46.
75. Almeida-Dias ER, Woiciechovski E. Ocorrência da Phagicola longa (Trematoda: Heterophyidae) em mugilídeos e no homem. Registro e Cananéia, SP. Hig Alim 1994;8:43-46.
76. Citti AL, Ribeiro NAS, Telles EO, Balian SC.
Ascocotyle ( Phagicola) longa parasitando tainhas ( Mugil liza, Valenciennes, 1836) em São Paulo: ocorrência, importância na saúde pública e estratégias de controle. Rev Ed Cont Med Vet Zoot CRMV-SP 2014;12:36-43.  77. Barros LA, Arruda VS, Gomes DC, Magalhães R. First natural infection by Ascocotyle ( Phagicola) longa Ransom (Digenea, Heterophyidae) in an avian host, Ardea cocoa L. (Aves, Ciconiformes, Ardeidae) in Brazil. Rev Bras Zool 2002;19:151-155.   78. Brandão M, Luque JL, Scholz T, Kostadinova A. New records and descriptions of digeneans from the Magellanic penguin Spheniscus magellanicus (Forster) (Aves: Sphenisciformes) on the coast of Brazil. Syst Parasitol 2013;85:79-98.    79. Jordan HE, Maples WP. Third record of Phagicola longa (Ransom, 1920) (Trematoda: Heterophyidae) in dogs from the United States. J Parasitol 1966;52:362-363.  80. Mello EBF, Carvalho Maugê G, Campos MS, Rocha UF, Dell’Porto A. Distribuição de helmintos do gênero Ascocotyle Looss, 1899 (Trematoda Fascioloidea Heterophyidae - Ascocotylinae) no tubo gastrointestinal de cão. Rev Fac Med Vet Univ São Paulo 1977;14:239-242.  81. Manfredi MT, Oneto M.
Phagicola longa (Heterophyidae) in dogs in Chile: morfological findings and taxonomical problems. Parasitología 1997;39:9-11.
82. Costa HMA, Lima WS, Costa JO.
Phagicola arnaldoi (Travassos, 1928) Travassos, 1929 (Trematoda, Heterophyidae) em Canis familiaris
. Arq Bras Med Vet Zootec 1988;36:591-595.
83. Costa JO, Guimaräes MP, Lima WS, Lima EA. Frequencia de endo e ectoparasitos de cães capturados nas ruas de Vitoria, ES, Brasil. Arq Bras Med Vet Zootec 1990;42:451-452.
84. Freitas JT, Ibáñez N, Córdova E. Ocurrencia de Phagicola arnaldoi en perros de Arequipa, Perú. Rev Per Med Trop 1972;1:55-57.
85. Chieffi PP, Leite OH, Dias RM, Torres DM, Mangini AC. Human parasitism by Phagicola sp. (Trematoda-Heterophyidae) in Cananéia, São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo 1990;32:285-288.    86. Chieffi PP, Gorla MC, Torres DM, Dias RM, Mangini AC, Monteiro AV, Woiciechovski E. Human infection by Phagicola sp. (Trematoda, Heterophyidae) in the municipality of Registro, São Paulo State, Brazil. J Trop Med Hyg 1992;95:346-348.  87. Antunes SA, Almeida-Dias ER.
Phagicola longa (Trematoda: Heterophyidae) em mugilídeos estocados resfriados e seu consumo cru em São Paulo, SP. Hig Alim 1994;8:41-42.
88. Knoff M, Luque JL, Amato JF. Community ecology of the metazoan parasites of grey mullets, Mugil platanus (Osteichthyes: Mugilidae) from the Littoral of the State of Rio de Janeiro, Brazil. Rev Bras Biol 1997;57:441-454.  89. Conceição JCS, São Clemente SC, Matos E. Ocorrência de Phagicola longus (Ransom, 1920) Price, 1932 em tainhas (Mugil sp.) comercializadas em Belém, Estado do Pará. Rev Bras Cienc Agr 2000;33:97-101.
90. Oliveira SA, Blazquez FJH, Antunes SA, Maia AAM. Metacercárias de Ascocotyle ( Phagicola) longa Ransom, 1920 (Digenea: Heterophyidae), em Mugil platanus, no estuário de Cananéia, SP, Brasil. Cienc Rural 2007;37:1056-1059.   91. Gueretz JS, Moura AB, Martins ML, Souza AP. Estudo da prevalência de Ascocotyle ( Phagicola) longa em mugilideos capturados na Baía da Babitonga, Santa Catarina, Brasil. Arch Vet Sci 2019;24:79-87.  93. Coelho MRT, São Clemente SC, Gottshalk S. Ação de diferentes métodos de conservação na sobrevivência de metacercárias de Phagicola longus (Ranson, 1920) Price, 1932, parasito de mugilídeos capturados no litoral do Estado do Rio de Janeiro. Hig Alim 1997;11:39-42.
94. Rodrigues MV, Pérez ACA, Machado TM, Orisaka FM, Kurissio JK, Lafisca A. Research of Ascocotyle ( Phagicola) longa in heat treated fillets of mullets ( Mugil platanus). Fish Aquac J 2015;6:115.  95. Borges JN, Lopes KC, Santos CP. Viability of Ascocotyle ( Phagicola) longa (Trematoda: Heterophyidae) metacercariae from mullets ( Mugil liza) from Rio de Janeiro, Brazil after exposure to freezing and heating in the temperature range from −35°C to 180°C. Food Control 2018;89:117-122.  96. Ostrowski de Núñez M. Estudio sobre estadios larvales de trematodes Digeneos de peces Cyprinodontiformes. Physis 1974;33:45-61.
97. Drago FB, Lunaschi LI. Update of checklist of digenean parasites of wild birds from Argentina, with comments about the extent of their inventory. Neotrop Helminthol 2015;9:325-350.
98. Boero JJ, Led JE, Brandetti E. Algunos parásitos de la avifauna Argentina. Analecta Vet 1972;4:17-34.
99. Alda P, Martorelli SR. Trematodes infecting the South-American intertidal mud snail Heleobia australis (Rissooidea: Cochliopidae). Acta Parasitol 2014;59:50-67.    100. Lunaschi LI, Cremonte F, Drago FB. Checklist of digenean parasites of birds from Argentina. Zootaxa 2007;1403:1-36.  101. Arruda VS, Muniz-Pereira LC, Pinto RM.
Ascocotyle ( Phagicola) rara sp. n. (Digenea, Heterophyidae) from Ixobrychus exilis (Aves, Ardeidae) in Brazil. Rev Bras Zool 2002;19:145-149.   102. Arruda VS, Pinto RM, Muniz-Pereira LC. New host and geographical records for helminths parasites of Ardeidae (Aves, Ciconiiformes) in Brazil. Rev Brasil Zool 2001;18:225-232.   103. McNeil R, Díaz MT, Casanova B, Villeneuve A, Thibault M. Trematode infestation as a factor in shorebird oversummering: a case study of the greater yellowlegs (Tringa melanoleuca). Bull Scand Soc Parasitol 1996;6:114-117.
104. Mcneil R, Díaz MT, Casanova B, Villeneuve A. Trematode parasitism as a possible factor in over-summering of greater yellowlegs (Tringa melanoleuca). Ornitol Neotrop 1995;6:57-65.
105. Digiani MC. Digeneans and cestodes parasitic in the white-faced íbis Plegadis chihi (Aves: Threskiornithidae) from Argentina. Folia Parasitol 2000;47:195-204.    106. Pérez ACA, Machado TM, Lopes RG, Okumura MPM, Rodrigues MV, Corrêa AM, Montano AP, São Clemente SC. Aspectos parasitológicos do pescado comercializado na costa da mata atlântica. Hig Alimentar 2012;26:120-124.
107. Carrera-Játiva PD, Rodríguez-Hidalgo R, Sevilla C, Jiménez-Uzcátegui G, Boero JJ, Led JE, Brandetti E. Gastrointestinal parasites in the Galápagos penguin Spheniscus mendiculus and the flightless cormorant Phalacrocorax harrisi in the Galápagos Islands. Mar Ornithol 2014;42:77-80.
108. Pinto HA, Mati VLT, Melo AL. Metacercarial infection of wild Nile tilapia ( Oreochromis niloticus) from Brazil. Sci World J 2014;2014:80749.   109. Lizama MAP, Takemoto RM, Pavanelli GC. Ecological aspects of metazoan parasites of Astyanax altiparanae Garutti e Britski, 2000 (Characidae) of the upper Paraná River floodplain, Brazil. Bol Insti Pesca 2008;34:527-533.
110. Torres P, Hott A, Boehmwald H. Protozoa, helminths and arthropods in cats of Valdivia City and their importance to man. Arch Med Vet 1972;4:20-29.
111. Pinto HA, Mati VLT, Melo AL. New records and a checklist of trematodes from Butorides striata (Aves: Ardeidae). Rev Mex Biodivers 2013;84:1100-1110.  112. González-Acuña D, Lohse E. Parasites of the American Kestrel ( Falco sparverius) in South-central Chile. J Raptor Res 2011;45:188-193.  113. Moreno L, González-Acuña D. Los parásitos de las aves rapaces de Chile: una revision. Bol Chil Ornitol 2015;2:93-102.
114. González-Acuña D, Cerda F, López J, Ortega R, Mathieu C, Kinsella M. Checklist of the helminths of the kelp gull, Larus dominicanus (Aves: Laridae), with new records from Chile. Zootaxa 2009;2297:27-43.  115. Brandão ML, Moreira J, Luque JL. Checklist of Platyhelminthes, Acanthocephala, Nematoda and Arthropoda parasitizing penguins of the world. Check List 2014;10:562-573.  116. Drago FB. Community structure of metazoan parasites of silverside, Odontesthes bonariensis (Pisces, Atherinopsidae) from Argentina. Iheringia Ser Zool 2012;102:26-32.   117. Santos CP, Simões SBE, Barbosa HS, Scholz T. Redescription of Ascocotyle ( Ascocotyle) felippei Travassos, 1928 (Digenea: Heterophiydae) with new synonymies. J Parasitol 2007;93:1468-1475.   118. Zdzitowiecki K, Niewiadomska K, Drozdz J. Trematodes of birds and mammals in the environs of H. Arctowski Station (South Shetlands, Antarctic). Acta Parasitol 1989;34:247-257.
119. Lunaschi LI, Cremonte F, Drago F. Checklist of digenean parasites of birds from Argentina. Zootaxa 2007;1403:1-36.  120. Díaz MT, Gómez E, Bashiryllah AK, Guilarte DV.
Ascocotyle nana Ransom, 1920 (Trematoda: Heterophyidae) from Venezuela and notes on its life cycle. Saber 2017;29:788-793.
121. Boero JJ, Led JE. El parasitismo de la fauna autóctona. III. Los parásitos de las aves argentinas. Rev Fac C Vet, La Plata 1968;10:97-129.
122. Boero JJ, Led JE, Brandetti E. El parasitismo de la fauna autóctona. Rev Fac Agro Vet, Buenos Aires 1972;1:17-29.
123. Drago FB, Lunaschi LI. Digenean parasites of ciconiiform birds from Argentina. Rev Mex Biodivers 2011;82:77-83.  125. Travassos L. Sur une nouvelle espèce du genre Pygidiopsis, Pygidiopsis pindoramensis n. sp. (Trematoda). Compt Rend Soc Bresil Biol (Paris) 1928;100:956-957.
126. Travassos L. Revisão do genero Ascocotyle Looss, 1899. Mem Inst Oswaldo Cruz 1930;23:61-97.
127. Rietschl G, Werding B. Trematodes of birds from northern Columbia. Z Parasitenkd 1978;57:57-82.
128. Ortubay SG, Semenas LG, Ubeda CA, Quaggiotto AE, Viozzi GP. Catalogo de peces dulceacuicolas de la Patagonia Argentina y sus parasitos metazoos. Río Negro, Argentina. Dirección de Pesca. 1994, pp 1-110 (in Spanish)..
129. Coelho MRT, São Clemente SC, Gottshalk S. Ação de diferentes metodos de conservacao na sobrevivencia de metacercarias de Phagicola longus (Ransom, 1920) Price, 1932, a parasite of mullet caught on the coast of the state of Rio de Janeiro. Revista Higiene Alimentar 1997;11:39-42.
130. Torres P, Hott A, Boehmwald H. Protozoos, helmintos y artropodos en gatos de la ciudad de Valdivia y su importancia para el hombre. Arch Med Vet 1972;4:20-29.
131. Torres P, Ramos M, Carrasco L, Neuman M, Franjola R, Navarrete N, Figueroa L. Protozoos helmintos y artropodos parasitos del perro domestico en la ciudad de Valdivia, Chile. Bol Chil Parasitol 1974;29:18-23 (in Spanish)..  132. Oberg C, Franjola R, Leyán V. 1979. Helmintos del perro domestico Canis familiaris en la ciudad de Valdivia, Chile. Bol Chil Parasitol 1979;34:21-26 (in Spanish)..  133. Bargiela JF. Los parasitos de la lisa Mugil cephalus L., en Chile: sistematica y aspectos poblacionales (Perciformes: Mugilidae). Gayaiia Zool 1987;51:3-58.
134. Nasir P, Lemus de Guevara D, Díaz MT. Estudio sobre larvas de trematodos de agua dulce. XXIV. Ciclo vital parcial de Ascocotyle paratenuicollis sp. n. (Trematoda: Digenea). Acta Biol Venez 1970;7:1-4.
135. Nasir P, Diaz MT. Studies on freshwater larval trematodes. XXVII. Partial life cycle of Caiguiria anterouteria gen. n., sp. n. (Trematoda: Digenea). Proc Helm Soc Wash 1971;38:21-23.
136. Carnevia D, Mazzoni RA. A preliminary note on the parasitofauna of the lebranche mullet (Mugil liza, Val 1836) in Uruguay. Riv Ital Piscic Ittiop 1986;21:109-111.
137. Morgades D, Katz H, Castro O, Capellino D, Casas L, Benítez G, Venzal J, Moraña A. Fauna Parasitaria del Lobo Fino (Arctocephalus australis) y del León Marino (Otaria flavescens) (Mammalia, Otariidae) en la costa de Uruguay. In Menafra R, Rodríguez-Gallego L, Scarabino F, Conde D eds, Bases para la conservación y el manejo de la Costa Uruguaya. Montevideo, Uruguay. Vida Silvestre Uruguay. 2006, pp 89-96.
138. Pereira EM, Müller G, Secchi E, Pereira J Jr, Valente ALS. Digenetic trematodes in South American sea lions from southern Brazilian waters. J Parasitol 2013;99:910-913.   139. Conroy D, Cecarelli P, Almeida ER. Diseases and parasites detected in grey mullet (Mugiliidae) from coastal waters of Sao Paulo State, Brazil. 3 Juvenile silver mullet (Mugil curema Val., 1836) and lebranche mullet (Mugil liza Val., 1836). Riv Ital Piscic Ittiop 1986;21:153-156.
140. Antunes SA, Almeida Dias ER.
Phagicola longa (Trematoda: Heterophyidae) em mugilídeos estocados resfriados e seu consumo cru em São Paulo, SP. Hig Alimentar 1994;8:41.
141. Barros L, Amato S. Experimental infection of dogs with metacercariae of Phagicola longa (Ransom, 1920) Price, 1932. Rev Bras Paras Vet 1996;5:61-64.
142. Conroy D, Perez K. A report on the experimental infection of a smooth-headed capuchin monkey (Cebus apella) with metacercariae of Phagicola longa obtained from silver mullet (Mugil curema) viscera. Riv Ital Piscic Ittiop 1985;20:154-155.
143. Pozza A, Lima F, Haas ML, Lehmann Albornoz PC.
Clinostomum sp. (Digenea: Clinostomidae) and Ascocotyle sp. (Digenea: Heterophyidae): metacercariae with zoonotic potential in fishes from Tramandaí River basin, southern Brazil. Bol Inst Pesca 2018;44:105-109.  144. Yamada FH, Takemoto R, Pavanelli GC. Ecological aspects of ectoparasites from the gills of Satanoperca pappaterra (Heckel, 1840) (Cichlidae) from the upper Paraná River floodplain, Brazil. Acta Sci Biol Sci 2007;29:331-336.  145. Camargo AA, Pedro NHO, Pelegrini LS, Azevedo RK, Silva RJ, Abdallah VD. Parasites of Acestrorhynchus lacustris (Lütken, 1875) (Characiformes: Acestrorhynchidae) collected from the Peixe River, southeast Brazil. Acta Sci Biol Sci 2015;37:231-237.  146. Yamada FH, Santos LN, Takemoto RM. Gill ectoparasite assemblages of two non-native Cichla populations (Perciformes, Cichlidae) in Brazilian reservoirs. J Helminthol 2011;85:185-191.   147. Hernández-Orts JS, Montero FE, Juan-García A, García NA, Crespo EA, Raga JA, Aznar FJ. Intestinal helminth fauna of the South American sea lion Otaria flavescens and fur seal Arctocephalus australis from northern Patagonia, Argentina. J Helminthol 2013;87:336-347.   148. Hernández-Orts JS, Montero FE, Crespo EA, García NA, Raga JA, Aznar FJ. A new species of Ascocotyle (Trematoda: Heterophyidae) from the South American sea lion, Otaria flavescens, off Patagonia, Argentina. J Parasitol 2012;98:810-816.   149. Simões SBE, Scholz T, Barbosa HS, Santos CP. Taxonomic status, redescription, and surface ultrastructure of Ascocotyle ( Phagicola) pindoramensis n. comb. (Digenea: Heterophyidae). J Parasitol 2006;92:501-508.   150. Scholz T, Muniz-Pereira LC, Santos CP. Taxonomic status of Ascocotyle ( Phagicola) rara Arruda, Muniz-Pereira et Pinto, 2002 (Digenea: Heterophyidae). Folia Parasitol 2006;53:297-301.   
Fig. 1
Maximum likelihood reconstruction between sequences obtained by our research group (marked with an asterisk) and sequences of heterophyid species from the GenBank database, with the tree inferred from 18S rDNA and 28S rDNA data sets. The numbers on the tree branches represent the percentage of bootstrap resampling. 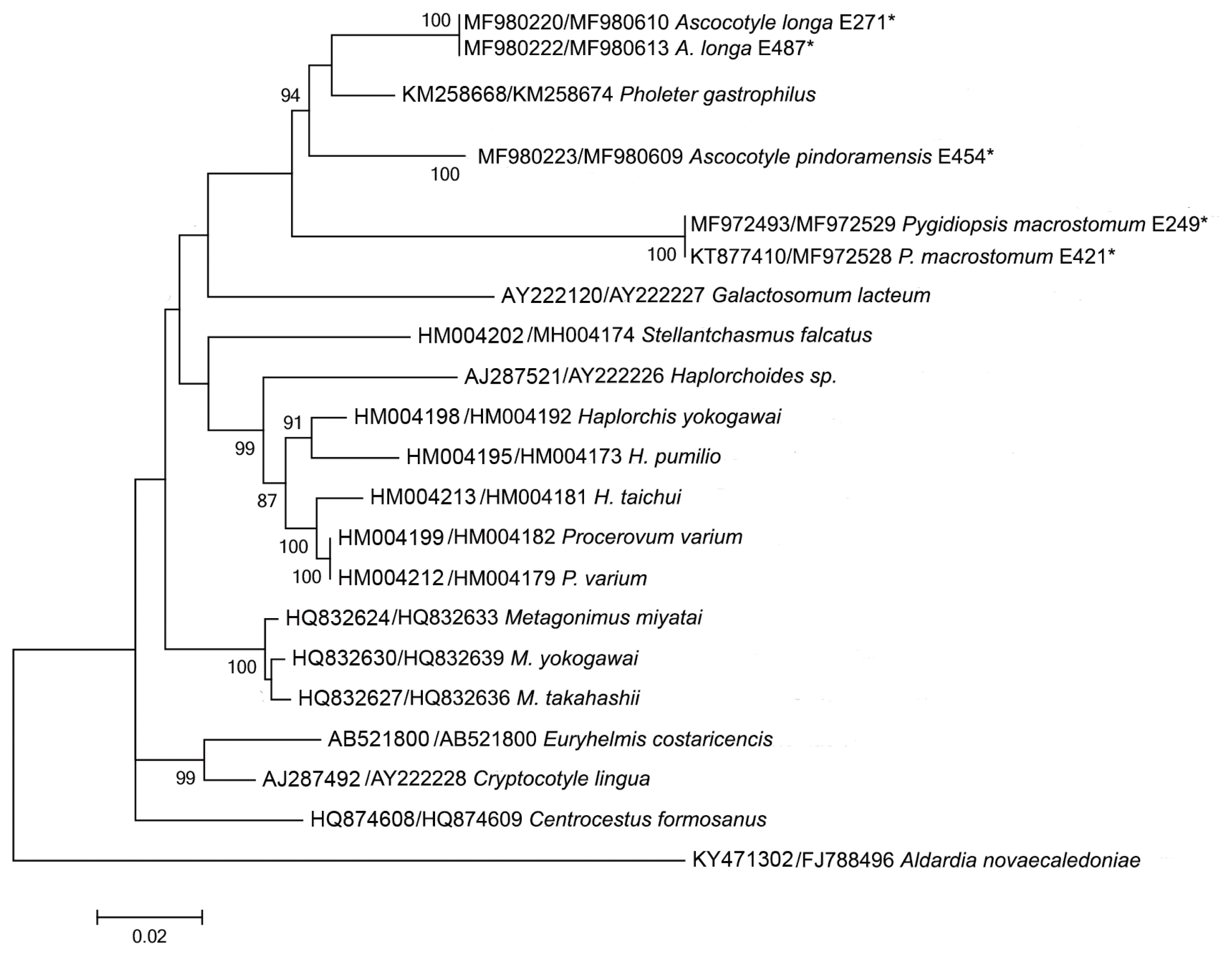
Fig. 2
Maximum likelihood reconstruction between sequences obtained by our research group (marked with an asterisk) and sequences of heterophyid species from the GenBank database, with the tree inferred from the mtDNA-cox1 data set. The numbers on the tree branches represent the percentage of bootstrap resampling. 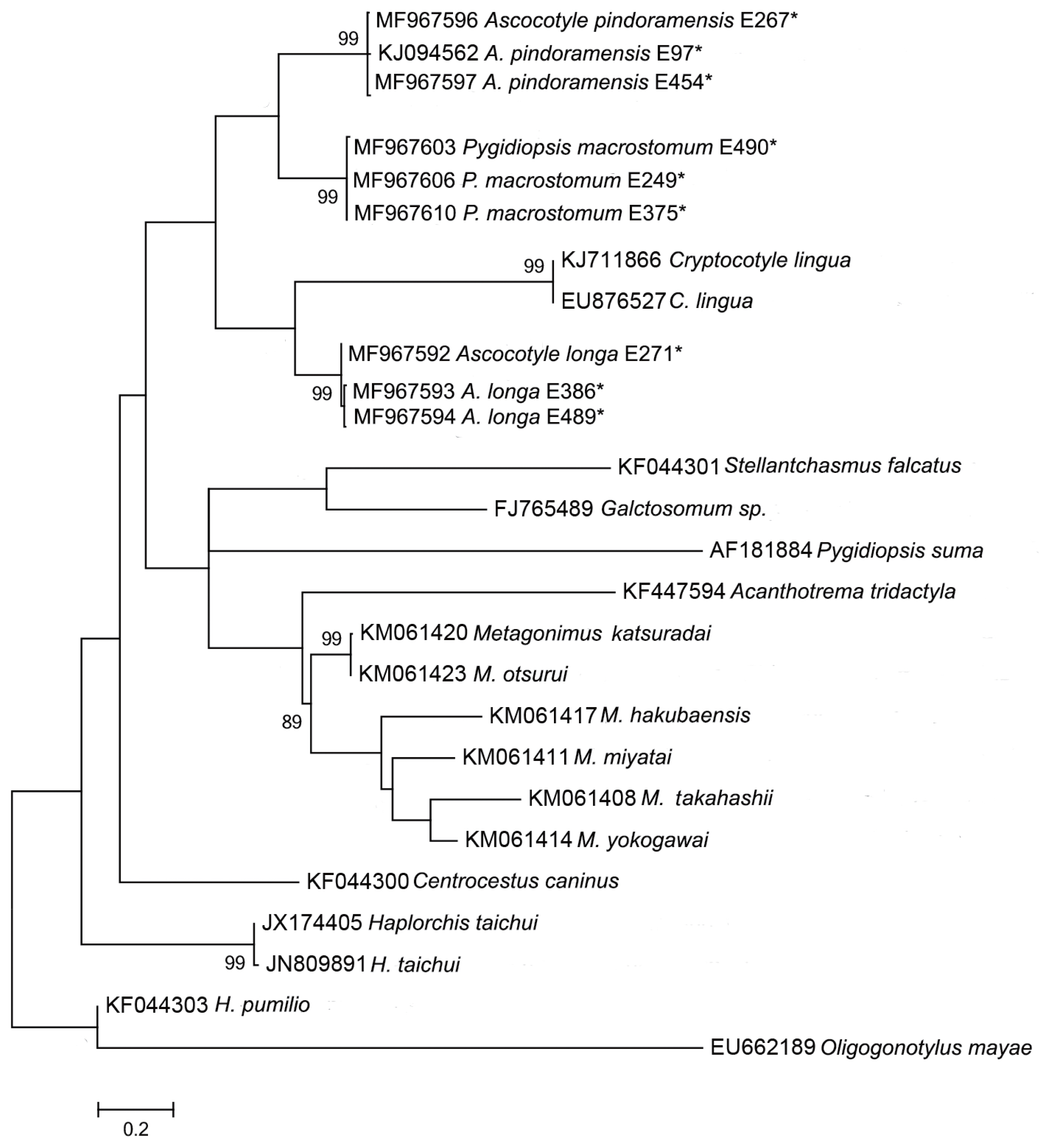
Table 1
The current list of species occurring in South America with their hosts and geographical distribution
|
Country |
Species |
1st Int. host |
2nd Int. host-site |
Definitive host |
References |
|
Argentina |
Ascocotyle angeloi
|
Heleobia castellanosae
|
Jenynsia lineata, Cnesterodon decemmaculatus (exp)-muscles, body cavity, internal organs, gill chamber |
Ixobrychus exilis; Gallus gallus (exp) |
[34,54] |
|
Argentina |
Ascocotyle angrense
|
|
Girardinus caudimaculatus (=Phalloceros caudimaculatus), Cnesterodon decemmaculatus (n, exp)-gills |
Platalea ajaja (=Ajaia ajaja), Ardea alba, Ardea alba egretta, Ardea cocoi, Ixobrychus involucris, Gallus gallus (exp) |
[33,96,98,121,122] |
|
Argentina |
Ascocotyle cameliae
|
|
|
Spheniscus magellanisus
|
[16] |
|
Argentina |
Ascocotyle diminuta
|
|
Jenynsia lineata, Odonthesthes bonariensis, Australoheros facetum (=Cichlasoma facetum), Cnesterodon decemmaculatus (exp), Gambusia affinis-gills |
Egretta thula, Ardea alba, Gallus gallus (exp) |
[33,118,123] |
|
Argentina |
Ascocotyle felippei (=A. tenuicollis) |
Heleobia piscium
|
Girardinus caudimaculatus (=Phalloceros caudimaculatus), Cnesterodon decemmaculatus (n, exp)-bulbus arteriosus |
Himantopus melanurus; Spheniscus magellanicus; Nycticorax nycticorax, Butorides striata, Ixobrychus involucris, Gallus gallus (exp), Mus musculus (exp), Anas platyrhynchus (exp) |
[33,36,42,96,98] |
|
Argentina |
Ascocotyle hadra
|
Heleobia parchappei
|
Jenynsia lineata, Gambusia affinis (exp), Cnesterodon decemmaculatus (n, exp)-liver and mesentery |
Plegadis chihi, Gallus gallus (exp), Mus musculus (exp) |
[6,35,105] |
|
Argentina |
Ascocotyle longa
|
Heleobia australis
|
Mugil liza-musculature, heart, mesentery |
Ardea alba
|
[9,17,27,97] |
|
Argentina |
Ascocotyle patagoniensis
|
|
Odonthesthes bonariensis, O. smitii-heart |
Otaria flavescens
|
[16,148] |
|
Argentina |
Ascocotyle secunda
|
Heleobia castellanosae, Heleobia parchappii
|
Cnesterodon decemmaculatus, Jenynsia lineata, Gambusia affinis (exp)-bulbus arteriosus |
Gallus domesticus (exp) |
[6] |
|
Argentina |
Ascocotyle secunda
|
|
Jenynsia lineata
|
Gallus gallus (exp) |
[6] |
|
Argentina |
Ascocotyle sp. |
|
Odonthesthes bonariensis-bulbus arteriosus, Percichthys trucha
|
Patagonina hatcheri
|
[117,128] |
|
Argentina |
Ascocotyle tertia
|
Heleobia castellanosae, Heleobia parchappii
|
Cnesterodon decemmaculatus, Gambusia affinis, Jenynsia lineata (n, exp)-bulbus arteriosus |
Gallus gallus (exp) |
[6] |
|
Argentina |
Cryptocotyle dominicana
|
|
Galaxias platei-fins, muscle |
|
[48] |
|
Argentina |
Cryptocotyle sp. |
|
|
Larus dominicanus
|
[55] |
|
Argentina |
Heterophyidae, metacercaria |
|
Percichthys trucha
|
|
[128] |
|
Argentina |
Pygidiopsis australis
|
|
Cnesterodus decemmaculatus
|
Gallus gallus (exp) |
[41,54] |
|
Argentina |
Pygidiopsis crassus
|
|
Jenynsia lineata, Cnesterodon decemmaculatus, Gambusia affinis
|
Himantopus melanurus, Gallus gallus (exp) |
[5,41,42,54] |
|
Argentina |
Pygidiopsis sp. |
|
|
Patagonina hatcheri
|
[128] |
|
Bolivia |
Cryptocotyle thapari
|
|
|
Lutra longicaudis
|
[53] |
|
Brazil |
Acanthotrema acanthotrema
|
|
|
Thalasseus maximus
|
[58] |
|
Brazil |
Ascocotyle pindoramensis
|
|
Poecilia vivipara, Phalloptychus januarius-Gill arches, musculature, mesenteries, intestinal wall, liver, gonads. |
Ixobrychus exilis, Nyctanassa violacea, Nycticorax nycticorax, Butorides striata, Mesocricetus auratus (exp) |
[37,57–59, 101,102,111, 125,126,149] |
|
Brazil |
Asccotyle angeloi (=A. rara) |
|
|
Ixobrychus exilis
|
[37,58,59,101,150] |
|
Brazil |
Ascocotyle angrense
|
|
|
Butorides striata, Butorides sp., Ixobrychus exilis, Ardea cocoi
|
[59,102,111] |
|
Brazil |
Ascocotyle diminuta
|
|
|
Butorides sp. |
[59] |
|
Brazil |
Ascocotyle felippei (=A. tenuicollis) |
|
Poecilia vivipara-heart bulb and and, more rarely, in the gills and mesentery |
Ixobrychus exilis., Leucophoyx sp., Phalacrocorax sp., ardeid birds |
[37,58,59,117] |
|
Brazil |
Ascocotyle longa (=A. arnaldoi) |
Heleobia australis
|
Mugil liza (=Mugil platanus), Mugil sp. -body musculature, heart, stomach, liver, kidney, spleen, gonads and mesentery |
Thalassarche melanophris (=Diomedea melanophrys), Thalassarche sp., Ardea cocoi, Spheniscus magellanicus, Canis lupus, Otaria flavescens, Rattus sp., Mesocricetus auratus (exp) |
[14,24,26,37,57–59,75–78,82,87–91,93,95,101,106, 127,138,139,141] |
|
Brazil |
Ascocotyle sp. (=Phagicola sp.) |
|
Mugil sp., Astyanax altiparanae, Astyanax spp. -bulbus arteriosus, Satanoperca papaterra, Cichla piquiti, C. kelberii-gills, Acestrorhynchus lacustris, |
|
[85,86,109,143–146] |
|
Brazil |
Centrocestus formosanus
|
Melanoides tuberculata
|
Oreochromis niloticus, Australoheros facetus
|
Butorides striata
|
[26,43,44,108,111] |
|
Brazil |
Cryptocotyle thapari
|
|
|
Pteronura brasiliensis
|
[52] |
|
Brazil |
Galactosomum cochlear
|
|
|
Sterna hirudinacea, S. sandivicensis, Larus dominicanus, Sula leucogaster
|
[57,58] |
|
Brazil |
Galactosomum cochleariforme
|
|
|
Fregata magnificens
|
[57,58] |
|
Brazil |
Galactosomum spinetum
|
|
|
Rhynchops nigra
|
[57,58] |
|
Brazil |
Heterophyidae gen. sp. |
Melanoides tuberculata
|
|
|
[46] |
|
Brazil |
Opisthometra planicollis
|
|
|
Sula leucogaster
|
[59] |
|
Brazil |
Pygidiopsis macrostomum
|
Heleobia australis
|
Poecilia vivipara, Phalloptychus januarius, Jenynsia multidentata-mesentery |
Rattus norvegicus, Mesocricetus auratus (exp) |
[4,8,40,58,125] |
|
Brazil |
Stictodora acanthotrema
|
|
|
Thalasseus maximus
|
[58,59] |
|
Chile |
Ascocotyle felippei
|
|
|
Falco sparverius
|
[112,113] |
|
Chile |
Ascocotyle longa
|
|
Mugil cephalus-mesentery, liver, heart |
Dogs, birds and mammals, Mus musculus (exp) |
[81,130–133] |
|
Chile |
Ascocotyle sp. (=Phagicola sp.) |
|
|
Cats |
[130] |
|
Chile |
Cryptocotyle sp. |
|
|
Larus dominicanus
|
[118,124] |
|
Colombia |
Ascocotyle longa
|
|
Poecilia reticulata, Xiphophorus helleri, X. maculatus-Mugil incilis
|
Birds and mammals |
[18] |
|
Colombia |
Centrocestus formosanus
|
Melanoides tuberculata
|
Andinoacara pulcher (=Aequidens pulcher) |
|
[49] |
|
Colombia |
Galactosomum cochleariforme
|
|
|
Marine birds |
[60] |
|
Colombia |
Galactosomum johnsoni
|
|
|
Birds |
[60,127] |
|
Colombia |
Galactosomum puffini
|
|
|
Sterna maxima, Egretta thula (=Leucophoyx thula) |
[127] |
|
Colombia |
Opisthometra planicollis
|
|
|
Sula leucogaster
|
[60] |
|
Ecuador |
Heterophyidae gen. sp. Eggs |
|
|
Phalacrocorax harrisi
|
[107] |
|
Peru |
A. longa (=A. arnaldoi) |
|
Mugil cephalus
|
Cairina moschata, birds and mammals, dogs, Gallus gallus (exp) |
[32,56,82–84] |
|
Peru |
Centrocestus formosanus
|
Melanoides tuberculata
|
|
|
[50] |
|
Peru |
Galactosomum sp. |
|
|
Larus pipixcan, Sterna hirundo
|
[56] |
|
Peru |
Pygidiopsis sp. |
|
|
Aequidens rivulatus
|
[56] |
|
Uruguay |
Ascocotyle longa
|
Heleobia australis
|
Mugil liza (=M. platanus) |
Arctocephalus australis, Otaria flavescens
|
[31,73,136,137] |
|
Venezuela |
Ascocotyle nana
|
|
Caquetaia kraussii-cranial cavity and musculature |
Gelochelidon nilotica, rats (exp) |
[120] |
|
Venezuela |
Ascocotile paratenuicollis
|
|
Poecilia reticulata (=Lebistes reticulatus) |
Gallus gallus (exp) |
[134] |
|
Venezuela |
Ascocotyle longicollis
|
|
|
Birds |
|
|
Venezuela |
Ascocotyle sp. |
|
Cyprinodontiform fish |
|
[62] |
|
Venezuela |
Centrocestus formosanus
|
|
Synbranchus marmoratus, Anablepsoides harti (=Rivulus harti) |
Anas platyrhynchos, Columba livia, Gallus gallus (exp) |
[51] |
|
Venezuela |
Haplorchis pumilio
|
Melanoides tuberculata (n, exp)-digestive gland |
Anablepsoides harti (=Rivulus harti)-muscular tissue under scales of caudal fin |
Butorides striatus, ducks (exp) |
[50,51] |
|
Venezuela |
Pholeter anterouterus
|
|
|
Phalacrocorax olivaceus
|
[61] |
|
Venezuela |
Pygidiopsis crassus
|
Snails-heart and hepatopancreas |
|
|
[62] |
|
Venezuela |
Pygidiopsis sp. (=Caiguiria anteroutenia) |
|
Poecilia reticulata (=Lebistes reticulatus) |
Himantopus himantopus, Gallus gallus (exp) |
[135] |
|
Venezuela |
Stictodira sp. |
|
|
Tringa melanoleuca
|
[103,104] |
Table 2
Sequences of Heterophyidae deposited in GenBank
|
Species |
mtDNA cox-1 |
18S rDNA |
28S rDNA |
5.8S rDNA and ITS2 region |
|
Ascocotyle longa
|
MF967591-MF967594 |
MF980220-MF980222 |
MF980610-MF980614 |
MF978362-MF978371 |
|
Ascocotyle pindoramensis
|
MF967595-MF967602 |
MF980223 |
MF980609 |
MF978372-MF978381 |
|
Pygidiopsis macrostomum
|
MF967603-MF967610 |
MF972488-MF972493 |
MF972527-MF972531 |
MF972292-MF972294, h1 |
|
|
|
|
MF972295-MF972296, h2
MF972297-MF972300, h3 |
|
|








