The gastrointestinal tract is continuously exposed to various pathogens, such as bacteria, viruses, and parasites, through food or water. Gastrointestinal pathogenesis evoked by these pathogens has been associated with anatomic and functional changes of the intestines [1]. Intestinal helminths, such as Nippostrongylus brasiliensis, have been suggested to be an important pathogen that can evoke intestinal changes, including neuronal damages [1,2]. It was suggested that intestinal inflammation during helminthic infections affects the plasticity of intestinal mucosal nerve fibers, including nerve density [2]. Moreover, if nerve degeneration occurs as a part of pathologic process in the gut, the mucosa must be reinnervated to restore normal functions [2]. However, intestinal neuronal damages and plasticity during intestinal trematode infections have never been reported.
Neodiplostomum seoulense is an intestinal trematode infecting humans and animals in the Republic of Korea and can cause acute enteritis in human infections [3]. This parasite is a good model to study intestinal pathogenesis and pathology since it can elicit severe mucosal inflammatory responses, including mastocytosis, goblet cell hyperplasia, macrophage infiltrations, and even death of the infected mice [4-6]. Mast-cell deficient W/Wv, their normal littermates +/+, C57BL/6, and C3H mice were all susceptible to death by day 23 post-infection (PI) with 200 metacercariae, with the exception of BALB/c mice, which were severely weakened but recovered after 28 days [4,7]. Their intestines showed severe anatomic and functional damage, highly suggestive of intestinal paralysis, which was irreversible in death-susceptible strains of mice [4]. Studies of N. seoulense infection using BALB/c mice would be useful for understanding mucosal pathogenesis and recovery during the course of infection.
Neuronal growth associated protein-43 (GAP-43) has been used to examine nerve fibers in the small intestine and suggested to be a good marker for detection of intestinal mucosal nerve plasticity [8,9]. GAP-43 immunoreactive nerve fibers are abundant in all layers of the small and large intestines [8,9]. Furthermore, all neuropeptide-immunoreactive fibers show GAP-43 reactivity [9]. Therefore, in the present study, GAP-43 immunoreactivity was examined in order to understand better the mucosal pathogenesis and recovery in N. seoulense-infected BALB/c mice.
BALB/c mice were purchased from the Orient Bio Animal Center (Seongnam, Gyeonggi-do, Korea). Mice were housed at room temperature (20-23℃) in a 12-hr light and 12-hr dark cycle with food and water ad libitum. Animal experiments were carried out strictly in accordance with the guidelines issued by our Institutional Animal Care and Use Committee, Seoul National University College of Medicine, Seoul, Korea.
The European grass snakes (Rhabdophis tigrina), naturally infected with N. seoulense metacercariae, were purchased from Hongchon, Gangwon-do, Korea. Metacercariae were collected by the artificial digestion method using 0.5% pepsin (1:10,000; Sigma-Aldrich, St. Louis, Missouri, USA) and 0.8% HCl at 37℃ for 2 hr [6]. Mice were infected orally with 200 or 500 metacercariae in 0.2 ml saline using a gavage needle. The infected mice were sacrificed every 7 days until day 35 PI, and their small intestines were resected.
The resected small intestines of N. seoulense-infected BALB/c mice were divided into the duodenum, jejunum, and ileum. The duodenum and jejunum were fixed in 10% neutral formalin and embedded in paraffin. Paraffin-embedded sections (7 µm thickness) were prepared for hematoxylin and eosin (H-E) and immunohistochemical (IHC) stainings. The specimens prepared by the above procedure were immersed in 10% neutral formalin for at least 8 hr. For immunohistochemistry, the paraffin-embedded sections of the small intestine were deparaffinized, rehydrated, incubated with a serum blocking solution, and stained with the primary GAP-43 antiserum diluted in bovine serum albumin (BSA)-PBS at 4℃ overnight. The serum dilution was 1:500 for anti-mouse GAP-43 antibody (Abcam, Cambridge, UK) as the primary antibody. After incubation with the primary antibody, the slides were stained using an anti-rabbit ABC staining kit (Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA) according to an avidin-biotin system. Counterstaining was performed with Mayer's hematoxylin solution (Wako, Osaka, Japan). The myenteric plexuses (red-brown in color), as visualized by 3, 3'-diaminobenzidine (DAB) staining, were counted using a light microscope, and the distribution of nerve fibers in the small intestines was determined, particularly in the duodenum and jejunum, which are the main habitat of N. seoulense [6]. The myenteric plexus was located between the longitudinal and circular muscle layers of the intestines. DAB-stained nerve fibers were counted regardless of the size and density of the myenteric ganglion. The counting of GAP-43 positive myenteric plexuses was done on 4-5 mouse samples every 7 days during 35 days PI.
The Student's t-test was used to determine the statistical significance of the results. A value of P<0.05 was regarded as significant. The statistics programs used were SPSS 12.0K and SigmaPlot 10.0.
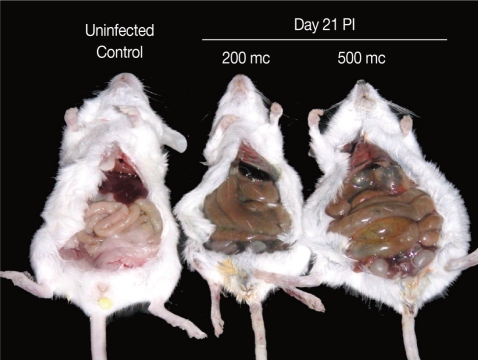
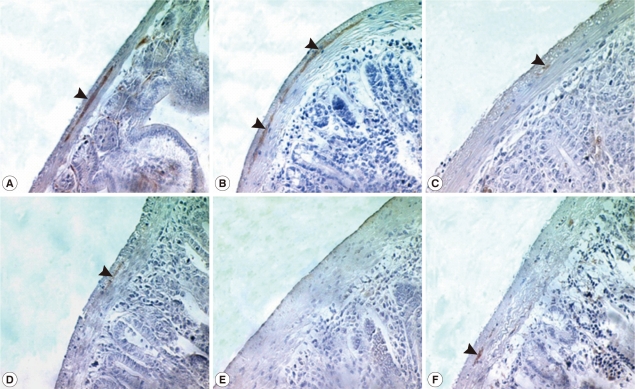
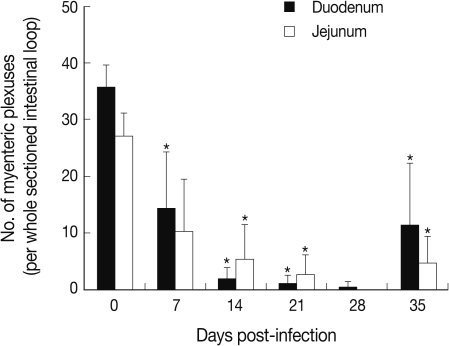
The abdomen of N. seoulense-infected mice became markedly distended and swollen. When it was opened, the intestinal loop appeared to be severely balooned and dilated (Fig. 1). In intestinal sections, GAP-43-labeled myenteric plexuses were found to be located between the longitudinal and circular muscle layers of the intestine, appearing as red-brown color by DAB staining (Fig. 2). The myenteric plexuses were well developed in the duodenum (35.8 in number) and jejunum (27.0) of uninfected control mice (Fig. 2), but in N. seoulense-infected mice, the plexuses decreased in number and diminished in size with progression of the infection (Fig. 2). The decrease was so remarkable that there were virtually no GAP-43 stained myenteric plexuses remained in the duodenum and jejunum at day 28 PI, 0.4 and 0.0 per whole cross-sectioned intestinal wall, respectively (Fig. 3).
It is well known that enteric pathogens can induce significant anatomic and functional damages in the host intestine accompanied by variable clinical symptoms, including abdominal pain, diarrhea, and fatigue [1,3,6,7]. Intestinal helminths also induce a variety of gross and histopathologic changes, including mucosal atrophy, goblet cell hyperplasia, mastocytosis, macrophage infiltrations, intestinal contraction or dilatation, progressive fibrosis, and even intestinal paralysis [2,4,6,7]. In N. brasiliensis infection, an early degenerative phase with decreased intestinal nerve densities and a later regenerative phase characterized by remodelling of these nerve fibers were observed [2]. However, involvement of intestinal nervous system in intestinal trematode infections remained unknown.
The present study focused on N. seoulense infection in mice because this parasite has been well-studied with respect to intestinal pathogenesis, including inflammatory responses and anatomic, histopathologic, and immunopathologic changes [4,6,7,10]. We observed the changing patterns of GAP-43 immunoreactive nerve expression in the myenteric plexus of the infected small intestines of BALB/c mice. According to our previous study, BALB/c mice are less susceptible to death of the host compared with other mouse strains although they have high worm burdens [4,7]. When infected with 200 metacercariae, BALB/c mice were severely weakened until days 23-28 PI but recovered after day 28 PI [4]. Therefore, BALB/c mice were considered to be a useful model to observe degeneration and regeneration of mucosal innervations during the course of infection.
We selected GAP-43, also known as B-50, as a marker for detection of mucosal nerve plasticity [8,9]. GAP-43 is a 5.1 kDa neuron-specific membrane-bound phosphoprotein located in the synaptosomal plasma protein where it plays a role in axonal growth and synaptic plasticity [11,12]. The presence of GAP-43 is well-known in the mammalian enteric nervous system, and dense GAP-43-like immunoreactivity is localized in nerves throughout the wall of the small intestine [8,13]. In humans, GAP-43 is a superior marker for evaluation of enteric nerve fibers, although other markers, including PGP 9.5, synaptophysin, and neuron-specific enolase, are also available [9]. In mice, GAP-43 knockout mice and their normal littermates have been used for brain and neurological researches [14,15]. GAP-43 was used also in a study examining changes in the enteric nervous system in N. brasiliensis infection, in which early degenerative and later regenerative phases occur [2].
Our results showed that the number of GAP-43 positive myenteric plexuses decreased significantly as the infection progressed to day 28 PI. Its level at day 28 PI was almost 0 in both structural parts of the small intestine. However, the number of GAP-43 positive myenteric plexuses was restored slightly toward normal levels at day 35 PI. The infected intestines showed marked balooning, dilatation, and degeneration until day 28 PI but this also began to normalize thereafter. These results corresponded well with our previous studies in which the intestinal pathology was progressively severe from infection until day 28 PI, and decreases in worm burdens, recovery of intestinal integrity, and activation of protective immunity occurred from day 35 PI [6,7,10].
Although N. seoulense infection results in impaired GAP-43 expression in the infected mouse intestines, it remained to be determined whether the neuronal damage is directly caused by the parasites or it is a secondary change in the damaged mucosa. The correlation between intestinal loop dilatation and depressed GAP-43 expression also remains to be elucidated. In addition, involvement of mucosal immune responses of the host, including immunopathogenesis and malfunctioning of the immune system (immune cells, mediators, antibodies, and cytokines), should be ruled out. It would also be interesting to study the intestinal pathogenesis and mucosal GAP-43 expression in infections with other intestinal trematodes, for example, Metagonimus yokogawai and Echinostoma hortense [3]. Through this study, GAP-43 immunostaining has been proved to be a useful tool for evaluating synaptic plasticity in myenteric plexuses during intestinal trematode infections.