AbstractIt is known that physicochemical conditions (e.g., pH, temperature, and ionic strength) affect the size of trichomonads.
In this study, the sizes of 4 isolates of Trichomonas vaginalis cultured for more than a year (called "old T") and 3 isolates freshly isolated from vaginitis cases (called "fresh T") were compared by scanning electron microscopy. Although the fresh T had shorter body length, body width, and flagellar length than old T, total length (about 26 µm), including body length, flagella length, and axostyle length was almost the same in the 2 groups. A striking difference was observed between
the axostyles of the 2 groups; the axostyle length of the fresh T (8.2 µm) was more than twice as long as that of the old T (4.0 µm). However, in several parasitology textbooks, the length of T. vaginalis is said to vary widely from 7 to 32 µm, and its undulating membrane is said to extend about half way (53.5%) to the posterior end of the body. On the other hand, in our study, the undulating membrane was observed to extend more than 3/4 of the body length (72.1%) in old T, whereas in fresh T it could not be measured. Taken together, we suggest that T. vaginalis averages 26 (21-32) µm in total length, with 9.5 (7.4-11.4) µm of body length and 6.8 (5.3-7.7) µm of width, and its undulating membrane extending 3/4 of its body length. Therefore, these findings may provide useful information for morphological characteristics of T. vaginalis.
Physicochemical conditions (e.g., pH, temperature, oxygen tension, and ionic strength) and ingredients of the medium affect the dimensions of Trichomonas vaginalis; Winston [1] reported that the variation in size of T. vaginalis was due to the effects of the host environment. Moon et al. [2] and Kim et al. [3] reported that trophozoites cultured at unfavorable pH in TPS-1 medium were significantly larger than those cultured at pH 7.0 and were less pathogenic. Honigberg and Brugerolle [4] reported that physicochemical conditions affected the shape of trichomonads. The size of T. vaginalis therefore may be expected to differ between trichomonads cultured for a long time and freshly isolated trophozoites.
We performed this study to observe size difference in scanning electron micrographs between trichomonads grown for more than a year (called "old T") and T. vaginalis (called "fresh T") freshly isolated from cases of vaginitis. In addition, we compared the size of T. vaginalis (called "schematic T") obtained from schematic drawings in parasitology and related books with those of old T and fresh T. Finally, we suggested the dimensions of T. vaginalis as measured by scanning electron microscopy in this study.
A total of 7 T. vaginalis isolates were used. Isolates KT4, KT9, and KT-Kim were collected from Koreans with vaginitis, and isolate 50148 was purchased from ATCC (Manassas, Virginia, USA). These 4 isolates had been cultured for more than a year in TYM (trypticase-yeast extract-maltose, pH 6.2) medium. In addition, 3 isolates of T. vaginalis were freshly obtained from women with vaginitis; they were prepared for scanning electron microscopy (SEM) immediately after they were collected. SEM was used to detect any superficial morphological differences between the trichomonads cultured for more than a year and the freshly isolated trophozoites. T. vaginalis isolates were fixed in 3% glutaraldehyde in 0.13 M Millonig's phosphate buffer (0.13 M NaH2PO4, 0.1 M NaOH, pH 7.3) at 4℃. They were then washed 3 times with PBS, allowed to adhere to poly-L-lysine-coated glass coverslips, post-fixed for 1.5 hr at room temperature in 1% OsO4 in Millonig's phosphate buffer, dehydrated in ethanols, dried in a critical point dryer, and mounted on stubs. They were then coated with gold using an ion sputtering coater, and observed with a scanning electron microscope (S-2380N, Hitachi, Tokyo, Japan) at an accelerating voltage of 25 kV. Fifteen trophozoites from the 4 isolates grown for more than a year and 27 trichomonads from the 3 fresh isolates were observed and measured. The size of the external (superficial) organelles of each trophozoite, namely length and width of body, length of the 4 anterior flagella, distance of axostyle protrusion from the end of the body, was measured. In addition, we measured the length of body, flagella, and axostyle in schematic drawings of T. vaginalis (called "schematic T") in 7 parasitology and related textbooks [5-11]. The ratios of flagella, body width, and axostyle length to body length were measured and compared with those of old T and fresh T.
The body length and width of fresh trichomonads (average L×W; 8.5×5.7 µm) were less than those of old trichomonads (9.5×6.8 µm). However, their ratios of width to length (68.6% in fresh T vs 71.9% in old T) were similar. Flagella were slightly shorter (average 10.5 µm) in fresh samples than in old ones (average 11.5 µm), and the ratio of flagellar length to body length was lower (107.4%) in the former than the latter (123.5%). Axostyle length differed the most between fresh T (average 8.2 µm) and old T (average 4.0 µm). When axostyle length was calculated as a percentage of body length, fresh trophozoites had significantly higher ratios (100.9%) than old trichomonads (40.5%) (Fig. 1). The total length including body, flagella, and axostyle was almost the same in the 2 groups (fresh T; 26.3 µm vs old T; 26.0 µm). The ratio of the length of undulating membrane (UM) to body length in old trichomonads was 72.1%, and it was difficult to observe the undulating membrane in freshly isolated trophozoites. Therefore, the ratio of UMs could not be measured.
The ratio of body width to body length in the schematic trichomonads was 64.8%, lower than those of fresh trichomonads (68.6%) and old trophozoites (71.9%). The ratios of axostyle and flagella to body length in the schematic T were also lower than in the fresh and old T. A significant difference was also seen in the ratio of axostyle to body length in schematic T (30.4%) and fresh T (100.9%), and the ratio for the flagella of schematic T (90.9%) was significantly lower than that for old T (123.5%). The undulating membrane in the schematic T (ratio 53.5%) extended only about a half of the length of the body, compared with 72.1% in old T (Table 1).
The axostyle of T. vaginalis runs from the anterior towards the posterior end of the cell. It forms the capitulum in the anterior portion and a narrow tube in the posterior [12]. It may have 2 functions in trichomonads; 1) it may be a supportive entity, 2) it may participate in the cell division process, causing constriction of the nucleus during karyokinesis [13]. Although the axostyles of fresh T were twice as long as those of old T, it is hard to determine the specific role of the longer axostyles in the fresh T; the fresh T need to overcome the flushing effects of the normal fluid secretion of the vagina in order to survive. In those conditions, the axostyle may have to be strong to fulfill a supportive role. Honigberg and Brugerolle [4] suggested that the shape of cells grown in culture tends to be more uniform than that of those in vaginal secretions. Consistent with this, we found that old T varied less in length than fresh T.
It is known that trichomonads tend to increase in size under certain, usually unfavorable, condition. Old T was about 1 µm longer and wider than fresh T, although their ratios of width to length were similar. This difference was in accordance with previous reports in which the culture medium probably affected the size of trichomonads [2,3,14].
Since the ratio of the length of the UM to that of the body has often been considered an important parameter distinguishing the 3 species of human-infecting Trichomonas [15], the UM extends about 2/3 of the length of the cell in Trichomonas tenax; and about 1/3 the length of the cell in T. vaginalis, while in Trichomonas hominis, it extends the whole length of the body [9]. However, the ratio (in percent) of the length of the UM to that of the body in T. vaginalis was 62.1±10.3% based on data in Table III of Honigberg and King [16]. In the present study, old T (72.1±10.5%) had significantly longer UM extension ratios than those of the schematic T as given in several parasitology textbooks (53.5±11.4%). The UM of fresh T, however, could not be measured because of the presence of apparent pseudopodial extension of the cytoplasm, especially on the side of the UM. Therefore, we suggest that the UM of T. vaginalis extends about 3/4 of the length of the body.
Measurements of the total length of T. vaginalis should include the body length, flagellar length, and axostyle length. The detailed reports of the structure of T. vaginalis by Honigberg and Brugerolle [4] and Benchimol [13] did not mention the total length, whereas several parasitology textbooks quoted a wide range of size (7-32 µm in length) [5-11]. Fresh and old trichomonads in the present study had almost the same average length (about 26 µm). Hence, it needs to present the size of T. vaginalis with some approximation to real trophozoites. In summary, we suggest that the total length of T. vaginalis (including the body length, flagellar length, and axostyle length) averages about 26 (21-32) µm, its body measures 9.5 (7.4-11.4)×6.8 (5.3-7.7) µm, and its undulating membrane extends 3/4 of its body length.
ACKNOWLEDGMENTThis research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0067578).
REFERENCES1. Winston RML. The relationship between size and pathogenicity of Trichomonas vaginalis. J Obstet Gynecol Br Commonw 1974;81:399-404. PMID: 4598896.
2. Moon MS, Kim JJ, Min DY, Lee JM, Lee KT. Effect of pH values on the propagation, isoenzyme patterns and morphological changes of Trichomonas vaginalis. Yonsei J Med Sci 1983;16:164-177.
3. Kim DW, Kim JJ, Lee KT. Culturing conditions affecting the pathogenicity of Trichomonas vaginalis. Yonsei Rep Trop Med 1984;15:12-16.
4. Honigberg BM, Brugerolle G. In Honigberg BM ed, Structure. Trichomonads Parasitic in Human. 1990, New York, USA. Springer-Verlag. pp 5-35.
5. Belding DL. Textbook of Clinical Parasitology. 1952, 2nd ed. New York, USA. Appleton-Century. pp 147-160.
6. Walter Beck J, Davies JE. Medical Parasitology. 1981, 3rd ed. Missouri, USA. Mosby Company. pp 52-54.
7. Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. 1984, 9th ed. Philadelphia, USA. Lea & Febiger. pp 47-51.
8. Neva FA, Brown HW. Basic Clinical Parasitology. 1994, 6th ed. Connecticut, USA. Appleton & Lange. pp 40-44.
9. Bogitsh BJ, Carter CE, Oeltmann TN. Human Parasitology. 2005, 3rd ed. California, USA. Elsevier. pp 91-96.
10. John DT, Petri WA. Markell and Voge's Medical Parasitology. 2006, 9th ed. Missouri, USA. Saunders. pp 55-59.
11. Garcia LS. Diagnostic Medical Parasitology. 2007, 5th ed. Washington, USA. ASM press. pp 123-130.
12. Lee KE, Kim JH, Jung MK, Arii T, Ryu JS, Han SS. Three-dimensional structure of the cytoskeleton in Trichomonas vaginalis revealed new features. J Electron Microsc (Tokyo) 2009;58:305-313. PMID: 19386993.
14. Ryu JS, Choi R, Park SY, Park H, Min DY. Biological and biochemical modulationof Trichomonas vaginalis KT9 isolate after shifting of culture medium from TPS-1 into TYM. Korean J Parasitol 1998;36:255-260. PMID: 9868891.
15. Honigberg BM, Lee JJ. Structure and division of Trichomonas tenax. Am J Hyg 1959;69:177-201. PMID: 13649673.
16. Honigberg BM, King VM. Structure of Trichomonas vaginalis Donné. J Parasitol 1964;50:345-364. PMID: 14169529.
Fig. 1Scanning electron microscopy of T. vaginalis. Two fresh isolates of T. vaginalis (A, B) collected from vaginitis cases have longer axostyles than isolates KT9 (C) and KT4 (D) cultured for more than a year in TYM. 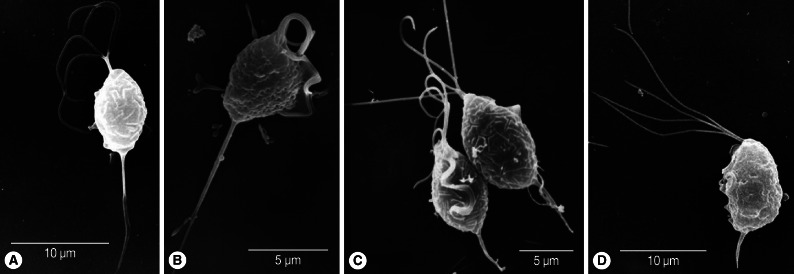
Table 1.Comparison of dimensions of cultured and freshly isolated Trichomonas vaginalis
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||