AbstractTick-borne pathogens can cause serious problems in grazing cattle. However, little information is available on tick-mediated diseases in cattle grazing on mountains. Thus, this study aimed to understand the potential problems related to tick-borne diseases in grazing cattle through the investigation of prevalent tick-transmitted infections, and their associated hematological changes, in terms of season and grazing type in Korean indigenous cattle (=Hanwoo). Hanwoo cattle from 3 regions of the Republic of Korea (=Korea) were either maintained indoors or placed on grassy mountains from spring to fall of 2014 and 2015. Cattle that grazed in mountainous areas showed a greater prevalence of tick-borne infections with an increased Theileria orientalis infection rate (54.7%) compared to that in non-grazing cattle (16.3%) (P<0.001). Accordingly, the red blood cell (RBC) count and hematocrit (HCT) values of grazing cattle were significantly lower than those of non-grazing cattle throughout the season (P<0.05). Moreover, RBC, hemoglobin (Hb), and HCT of T. orientalis-positive group were significantly lower than those of T. orientalis-negative group (P<0.05). T. orientalis is a widespread tick-borne pathogen in Korea. Grazing of cattle in mountainous areas is closely associated with an increase in T. orientalis infection (RR=3.4, P<0.001), and with consequent decreases in RBC count and HCT. Thus, these findings suggest that the Hanwoo cattle in mountainous areas of Korea are at a high risk of infection by T. orientalis, which can lead to hematological alterations. This study highlights the necessity of preventive strategies that target T. orientalis infection.
INTRODUCTIONHanwoo cattle (a traditional Korean indigenous breed: Bos taurus coreanae) are one of the most economically important species in the Republic of Korea (=Korea), as they are a significant source of nutrition for the Korean people [1]. Even though the demand for Hanwoo production has increased, most farms in Korea find it difficult to raise enough cattle due to the overcrowded conditions that are common in cattle farms of Korea. These overcrowded housing systems can cause multiple problems, such as financial burdens due to high feed prices, rapid spread of acute infectious diseases, restricted movement, and abnormal behavior, thus leading to poor animal welfare [2].
In order to resolve these issues, implementation of a pasturing system in mountainous areas is being considered in Korea. Although this type of grazing system reduces the production costs and improves animal welfare [3], it has several disadvantages in terms of nutritional management and disease control. Energy imbalances and seasonal temperature variations can compromise the health of the animal when the pasture-based system is used [4]. Higher exposure to ticks and the consequent risk of tick-borne disease in mountainous areas are also major concerns [5,6]. Global warming and ecological changes have resulted in favorable conditions for the rapid growth and reproduction of ticks over the recent decades. Multiplying tick populations and the frequent occurrence of tick-borne diseases can cause serious problems for cattle grazing, leading to economic losses for the livestock industry [7]. The aforementioned environmental changes and globalization may also give rise to the emergence of new species of tick vectors; therefore, new types of pathogens and the geographical distribution of tick-borne diseases should be continuously monitored all over the world, including Korea [8]. In addition, to ensure a safer mountain grazing system for cattle in Korea, research should be conducted on the potential problems associated with grazing [2].
Many Rickettsiae and Protozoa species (Bartonella spp., Ehrlichia spp., Rickettsia spp., Anaplasma spp., and Theileria/Babesia spp.) are transmitted by ticks [7]. Various tick-borne pathogens have been reported all over the world, including Korea; however, most of the previous studies have focused on detecting pathogens from ticks [9,10]. Even though tick-borne diseases are important in the grazing cattle industry [11], very little is currently known about the natural infection patterns and physiological changes associated with tick-borne pathogens in cattle that are pastured in mountainous areas [2,12]. Specifically, no prior studies have addressed the hematological changes induced in Hanwoo cattle by tick-borne pathogens in Korea except for a study in Holstein cattle [2].
Thus, the objective of the present study was to investigate the rate of infection with tick-borne pathogens and the hematological changes related to natural tick-borne diseases, in mountain-grazing Hanwoo cattle. This study is the first to report the hematological alterations induced by tick-borne pathogens in Hanwoo cattle, which is a valuable local breed of cattle in Korea. Our investigation provides beneficial evidence on preventive strategies against tick-borne pathogens, and will consequently help establish a successful cattle-grazing system in Korea.
MATERIALS AND METHODSEthics statementAll procedures were carried out according to the ethical guidelines for the use of animal samples, as approved by the Chonbuk National University Institutional Animal Care and Use Committee (IACUC), decision no. CBU 2014–00026. Verbal informed consent for the sampling of the cattle was obtained from the managers of the surveyed farms after the procedures and possible consequences of the study were explained.
Experimental designThe study was conducted on Korean indigenous cattle (Hanwoo) from 2014 to 2015 on 3 farms in the following, geographically distinct, locations in Korea: Hoengseong (southwest Gangwon Province), Jeongeup (southwest Jeolla Province), and central Jeju Island (Fig. 1). Hanwoo cattle aged 7–60 months were used in this study. Healthy Hanwoo cattle without any abnormal conditions, as determined through physical examination, were included in this study. The Hanwoo cattle were examined for infection with tick-borne pathogens in the spring (April; n=159), which was the starting point of the study. Subsequently, the cattle were either maintained indoors or placed on grassy mountains from spring to fall (April to October); we subsequently investigated the infection rate of tick-borne pathogens in the Hanwoo cattle. Control groups were randomly selected from among the cattle that had been raised indoors without pasturing; n=61 in summer (July to August) and n=25 in fall (October). The test groups were randomly selected from among the cattle that were allowed to graze on the mountains from spring to fall; n=28 in summer, n=25 in fall.
Sample collectionBlood samples were collected from the cattle housed indoors and those maintained outside on the pasture from spring to fall, as described in a previous report [2]. Briefly, we collected 5 ml of blood from the jugular veins of the cattle into EDTA-supplemented tubes; these were delivered to the lab immediately after collection. The samples were subjected to hematological analysis on the day of the blood collection; subsequently, they were immediately frozen at −80°C until DNA extraction. To identify hematological alterations that might have been caused by a tick-mediated infection, all the results were evaluated on an individual basis in terms of the season and grazing type.
PCR amplification and nucleotide sequencingPreviously described PCR primers and conditions [2] were used to screen for tick-borne pathogens (Theileria, Anaplasma, Ehrlichia, and Rickettsia spp.). Briefly, genomic DNA was extracted from 200 μl of whole blood using the DNeasy Blood & Tissue Kit (Qiagen Inc., Valencia, California, USA). The AccuPower Theileria PCR Kit (Bioneer, Daejeon, Korea) was used to detect Theileria, and the AccuPower Rickettsiales 3-Plex PCR Kit (Bioneer) was used to detect Anaplasma, Ehrlichia, and Rickettsia. All PCRs were performed using primer sets that targeted the genes for either 18S ribosomal RNA (rRNA; in the case of Theileria) or 16S rRNA (in the case of Anaplasma, Ehrlichia, and Rickettsia). The PCR products were subjected to 1.5% agarose gel electrophoresis and visualized using ethidium bromide staining. Blood samples positive for Theileria, Anaplasma, Ehrlichia, or Rickettsia were considered infected with tick-borne pathogens (Fig. 1).
In order to further confirm the presence of Theileria orientalis, a PCR was performed to detect the gene encoding the major piroplasm surface protein (MPSP) of T. orientalis in the Theileria-positive samples (Fig. 1) and the purified PCR products were sequenced, as described in our previous publication [13]. Briefly, the amplified DNA was purified using a QIAquick PCR Purification Kit (Qiagen) and sequenced (Bioneer). The DNA sequencing data were subjected to a Basic Local Alignment Scarch Tool (BLAST) analysis to determine closely corresponding sequences in the GenBank database. A phylogenetic tree based on nucleotide alignments was constructed using the neighbor-joining method [14]. Bootstrap analysis with 1,000 replications was conducted using MEGA version 6 [15].
Hematological analysisHematological parameters were measured using an automatic blood analyzer (Hemavet 960, Erba Diagnostics Inc., Miami, Florida, USA). Specifically, a red blood cell (RBC) profile included the RBC count, hemoglobin (Hb), hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC).
Statistical analysisStatistical analysis was performed using the SPSS 23.0 software package (SPSS, Chicago, Illinois, USA). Pearson’s chi-square test (χ2) was applied to evaluate whether Theileria infection was associated with the seasons and grazing types. Moreover, the relative risk (RR) of all factors was used to measure the strength of association. Point biserial correlation coefficient (r) was calculated to evaluate the relationship between hematological values and Theileria infection. To evaluate hematological alterations among season, grazing type and Theileria infection, quantitative data were analyzed using either a 2-tailed independent t-test or Mann-Whitney U test, depending on the results of a normality test. Data were expressed as mean±standard deviation (SD) and P-values<0.05 were considered significant.
RESULTSGeographical investigation of tick-transmitted infectionThe blood of Hanwoo cattle from 3 farms was examined for tick-borne pathogens before pasturing in the spring (Fig. 2). The rates of infection with tick-borne pathogens were 4.0% (2/50 cattle), 30.6% (15/49 cattle), and 25% (15/60 cattle) in the farms of Hoengseong, Jeongeup, and Jeju Island, respectively. Therefore, the average rate of infection with tick-borne pathogens was 20.1% (32/159 cattle) before the grazing started. Theileria was detected in all the farms surveyed in this study, and its infection rate was higher than that of the other tick-borne pathogens. Several Hanwoo cattle were infected with Rickettsia in Jeongeup and Jeju Island. Ehrlichia and Ana-plasma were not detected in the cattle before the grazing began.
Tick-borne infection in Hanwoo cattle by grazingTo investigate natural or epidemic infection with tick-borne pathogens in Hanwoo cattle raised in the mountainous areas of Korea, the rates of infection with tick-borne pathogens were compared between housed and pastured cattle from spring to fall (Fig. 3). The infection rate among the housed cattle was 17.6% (43/245 cattle), whereas that among the pastured cattle was 56.6% (30/53 cattle). Among the cattle that were positive for tick-borne pathogens, the percentages of Theileria-positive cattle were 93.0% (40/43 cattle) and 96.7% (29/30 cattle) in housed and pastured cattle, respectively. These figures included those that were co-infected with Anaplasma or Rickettsia. Theileria was the major tick-transmitted infection in Hanwoo cattle. In addition, Anaplasma was detected considerably more often among pastured cattle, 30% (9/30 cattle) including co-infected cattle, than among housed cattle, 2.3% (1/43 cattle).
Meanwhile, PCR detection of MPSP and phylogenetic analysis of the MPSP sequences revealed that the Theileria isolates constituted the T. orientalis species. Detailed information regarding this can be obtained from our previous publication [13]. The sequencing of Anaplasma and Rickettsia PCR samples was not processed.
The effect of season and grazing type on T. orientalis infection and hematological changesWe analyzed in detail the association between T. orientalis infection and the variables (season and grazing type). The seasonal T. orientalis infection rates of housed cattle were 18.2% (29/159 cattle) in the spring, 11.5% (7/61 cattle) in the summer, and 16.0% (4/25 cattle) in the fall; therefore, the average infection rate was 16.3% (40/245 cattle) over the whole season (Fig. 4A). A chi-square test did not reveal significant differences in the association between the season and infection rates among the housed cattle (χ2=1.5, P >0.05). In contrast, the T. orientalis infection rates of the pastured cattle were 46.4% (13/28 cattle) in the summer and 64.0% (16/25 cattle) in the fall, the average rate being 54.7% (29/53 cattle) throughout the whole season. There was a significant association between the season and infection rate in the pastoral environment (χ2=28.1, P <0.001). The T. orientalis-positive rate was 3.4 times higher among pastured cattle than among housed cattle throughout the whole season; RR=3.4, 95% confidence interval (CI)=2.3–4.9, and in both summer (RR=4.1, 95% CI=1.8–9.0) and fall (RR=4.0, 95% CI=1.6–10.3). This indicated a close relationship between the infection rate of T. orientalis and grazing type (χ2=36.1, P <0.001).
Subsequently, the seasonal and grazing effects on RBC parameters were evaluated (Fig. 4B) and compared to those on T. orientalis infection to investigate the association between hematological alterations and T. orientalis infection. The RBC count and HCT did not differ among the seasons (P >0.05). Pastured cattle showed a significantly lower RBC count and HCT compared to housed cattle in summer (P <0.01) and fall (P <0.05). The RBC (9.3±1.9 M/μl) and HCT (34.9±5.0%) of the pastured cattle were lower than the RBC (7.7±1.5 M/μl) and HCT (32.1±4.8%) of the housed cattle throughout the whole season (P <0.01), indicating that grazing was a risk factor for alterations in the hematological parameters.
Grazing throughout the season was closely associated with the increase in T. orientalis infection and the decreases in the RBC count and HCT. Correlation analysis suggests a negative relationship between T. orientalis infection and the hematological changes (r=−0.2, P <0.01). The changes in the hematological profiles by season and grazing type are listed in detail in supplementary Table 1.
T. orientalis infection-associated changes in RBC profile in Hanwoo cattleIn the present study, T. orientalis accounted for>90% of Hanwoo cattle infections by tick-borne pathogens; such infections may cause an economic loss to the Hanwoo cattle industry in Korea. To confirm whether T. orientalis infection induced alterations in the RBC parameters, RBC, Hb, and HCT values were compared between the groups that were negative and positive for T. orientalis infection (Fig. 5). The RBC, Hb, and HCT values obtained in the T. orientalis-positive group were 8.3±2.0 M/μl, 9.1±2.0 g/dl, and 32.2±5.2%, respectively, and were significantly lower than those obtained in the T. orientalis-negative group which were 9.2±1.8 M/μl, 10.0±2.0 g/dl, and 35.0±4.8%, respectively (P <0.01; Fig. 5). T. orientalis infection might have caused the change in the RBC profiles.
Further data were analyzed in terms of season and grazing type. They revealed that the RBC, Hb, and HCT values of the T. orientalis-positive group tended to be lower than those of the T. orientalis-negative group among all seasons and grazing types (Table 1). Housed cattle that were positive for T. orientalis showed dramatically lower RBC values than those that were negative for T. orientalis in the summer (P <0.05). The Hb was statistically lower in the T. orientalis-positive group than in the T. orientalis-negative group among housed cattle in the spring (P <0.01), and among pastured cattle in the fall (P <0.05). The HCT was also significantly lower among the T. orientalis-positive group than among the T. orientalis-negative group in housed cattle in the spring (P <0.05). This implied that T. orientalis contributed to hematological alterations, such as decreases in the RBC count, Hb, and HCT among Hanwoo cattle, regardless of the season and grazing type.
DISCUSSIONIn the present study, we investigated the tick-transmitted pathogens that are currently prevalent in Hanwoo cattle pastured on the grassy mountains of Korea during the summer and fall seasons. We also examined the association between T. orientalis infection and hematological parameters. This is first report to suggest that T. orientalis infection is closely associated with anemia in Hanwoo cattle as evidenced by decreased RBC, Hb, and HCT values.
Bovine theileriosis is a tick-borne haemoprotozoan disease caused by parasites of the genus Theileria in bovines. Theileria spp. are classified into 2 groups: lymphoproliferative Theileria spp. (T. parva and T. annulata) and non-lymphoproliferative Theileria spp. (known historically as T. sergenti and T. buffeli) [16]. In particular, T. annulata and T. parva cause high mortality and morbidity due to uncontrolled lymphocyte proliferation in tropical and subtropical regions of the world [17,18]. T. orientalis is responsible for benign/oriental theileriosis; it proliferates in erythrocytes and causes erythrocyte destruction [19,20]. Although T. orientalis is believed to have only mild or no pathogenicity in cattle, recent outbreaks of oriental theileriosis in the Asia-Pacific region have caused major concerns in the cattle industry due to serious problems such as reduced growth and production losses in infected cattle [21,22]. In Korea, T. orientalis is thought to be the causative agent of bovine theileriosis with a high prevalence of Haemaphysalis longicornis, which is a major biological vector of T. orientalis [23]. The present study indicated that infection of cattle with T. orientalis was more prevalent than infection by other tick-borne pathogens in all the farms investigated, and that the prevalence of T. orientalis infection increased after grazing. Similarly, isolation of Theileria parasites from ticks [10] and high rates of T. orientalis infection in grazing cattle have been reported previously in Korea [13].
In a previous study, H. longicornis, which is known to be a vector of disease agents, was the most common tick species observed in Korea [24]. Indeed, in our own recent report, no other tick species were found among the adult ticks that were collected from grazing Holstein cattle in Jiri Mountain, Korea [2]. Concordantly, in the present study, all ticks collected from the Hanwoo cattle pastured in Jeju Island, which is geographically distant from Jiri Mountain, were also H. longicornis (data not shown). This suggests that H. longicornis is a local and endemic tick vector capable of transmitting T. orientalis to grazing livestock and wild animals that inhabit the mountainous areas of Korea. In particular, pastured cattle may be highly exposed to T. orientalis through bites from H. longicornis; thus, they may be at a higher risk of infection with T. orientalis [25]. Therefore, the RBC and HCT decreases seen in grazing Hanwoo cattle might be due to bleeding caused by ectoparasite infestation or hemolysis of RBC caused by T. orientalis infection.
Besides T. orientalis, Anaplasma was detected among the pastured cattle in the present study, and the rate of infection was higher than that in the non-pastured cattle. Anaplasma is important as a zoonotic, tick-borne agent that causes both human and animal illness throughout the world, and several studies have reported Anaplasma isolation from ticks in Korea [7,9,24]. Nonetheless, as per our knowledge, cattle infection with Anaplasma has not been reported previously, although a Korean study did find A. phagocytophilum antibodies in the serum of Holstein cattle [26]. In the present study, we did not describe the physiological status and hematological abnormalities of Hanwoo cattle infected with A. phagocytophilum, because the sample number was too small. In future, the ability of A. phagocytophilum to cause anemia should be investigated further because bovine anaplasmosis is characterized by anemia and jaundice [27].
One major clinical manifestation of T. orientalis is anemia. Despite this, few studies have reported the evidence of anemia in cattle that have been experimentally or naturally infected with T. orientalis [2,28]. Moreover, to the best of our knowledge, no reports have clearly documented the changes in hematological parameters caused by natural infection of Hanwoo cattle with T. orientalis. Japanese indigenous cattle were reported to be more resistant to T. orientalis infection than Holstein [29]; similarly, Hanwoo cattle are likely to show a different sensitivity to T. orientalis. The rate of anemia was lower in Hanwoo cattle (5.9%) than Holstein cattle (26.6%) among T. orientalis-infected cases (data not shown). Although T. orientalis infection appeared to be associated with subclinical cases in Hanwoo cattle, this study suggests that T. orientalis may be responsible for causing anemia in Hanwoo cattle by causing a decrease in RBC, Hb, and HCT levels.
The MPSP shows significant sequence diversity and pathogenicity [11]. To date, 11 genotypes (types 1–8 and N1–N3) of T. orientalis have been identified based on the sequence of MPSP, and 2 of these genotypes (chitose [type 1] and ikeda [type 2]) are thought to be pathogenic [30]. Thus, it is important to conduct further studies to identify the correlation between the pathogenic types and clinical signs by determining the MPSP genotypes of T. orientalis that are related to hematological changes in Hanwoo cattle.
Interestingly, T. orientalis was observed among Hanwoo cattle that had never been placed on the pasture. We are unable to explain the cause or infection route of T. orientalis in housed cattle, but our results suggest that Hanwoo cattle in Korea were infected with T. orientalis, even though they are raised indoors. The majority of T. orientalis-infected cattle become chronic carriers of the parasite, occasionally developing severe or fatal anemia under certain unfavorable conditions [31]. Furthermore, prenatal infection due to intrauterine transmission of T. orientalis [32,33] may be a chronic risk factor that threatens cattle health from generation to generation. Although T. orientalis appears to have low virulence in healthy cattle, cattle with subclinical theileriosis in endemic regions become sources of infection for vector ticks as long-term carriers of piroplasm. Thus, latent infections are important in the epidemiology of theileriosis [17,34]. Even a small number of infected cattle can constitute a nidus of infection for a whole herd [12], and explosive outbreak of T. orientalis infection may cause considerable economic losses in Korea under various environmental changes such as global warming.
The current study revealed alterations in the RBC profile after T. orientalis infection in Hanwoo cattle. Specifically, T. orientalis-infected cattle had significantly lower RBC count, Hb, and HCT than uninfected cattle. We suppose that the increased rate of T. orientalis infection resulted from the propagation of active ticks in the mountainous areas in the summer and fall. Infection of cattle with T. orientalis may lead to economic losses in Hanwoo cattle farms in Korea. Therefore, measures to reduce tick bites and infestation must be introduced to prevent T. orientalis infection among cattle and to establish a successful grazing system in Korea.
Supplementary MaterialsSupplementary Table 1.Alterations in RBC and WBC parameters in terms of the season and grazing type ACKNOWLEDGMENTSThis work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (project no. PJ010092), which was funded by the Rural Development Administration of the Republic of Korea.
REFERENCES1. Lee SH, Park BH, Sharma A, Dang CG, Lee SS, Choi TJ, Choy YH, Kim HC, Jeon KJ, Kim SD, Yeon SH, Park SB, Kang HS. Hanwoo cattle: origin, domestication, breeding strategies and genomic selection. J Anim Sci Technol 2014;56:2.
2. Choi KS, Yu DH, Chae JS, Park BK, Yoo JG, Park J. Seasonal changes in hemograms and Theileria orientalis infection rates among Holstein cattle pastured in the mountains in the Republic of Korea. Prev Vet Med 2016;127:77-83.
3. Barkema HW, von Keyserlingk MA, Kastelic JP, Lam TJ, Luby C, Roy JP, LeBlanc SJ, Keefe GP, Kelton DF. Invited review: changes in the dairy industry affecting dairy cattle health and welfare. J Dairy Sci 2015;98:7426-7445.
4. Arnott G, Ferris CP, O’Connell NE. Review: welfare of dairy cows in continuously housed and pasture-based production systems. Animal 2017;11:261-273.
5. Kamio T, Ito Y, Fujisaki K, Minami T. Infection rates of Theileria sergenti in Haemaphysalis longicornis ticks collected from the field in Japan. Nihon Juigaku Zasshi 1990;52:43-48.
6. Onuma M, Kakuda T, Sugimoto C.
Theileria parasite infection in East Asia and control of the disease. Comp Immunol Microbiol Infect Dis 1998;21:165-177.
7. Kang SW, Doan HT, Choe SE, Noh JH, Yoo MS, Reddy KE, Kim YH, Kweon CH, Jung SC, Chang KY. Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol Int 2013;62:276-282.
8. Kamau J, de Vos AJ, Playford M, Salim B, Kinyanjui P, Sugimoto C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit Vectors 2011;4:22.
9. Doan HT, Noh JH, Choe SE, Yoo MS, Kim YH, Reddy KE, Quyen DV, Nguyen LT, Nguyen TT, Kweon CH, Jung SC, Chang KY, Kang SW. Molecular detection and phylogenetic analysis of Anaplasma bovis from Haemaphysalis longicornis feeding on grazing cattle in Korea. Vet Parasitol 2013;196:478-481.
10. Kang SW, Nguyen LT, Noh JH, Reddy KE, Kweon CH, Choe SE. Phylogenetic analysis of benign Theileria species based on major piroplasm surface protein (MPSP) genes from ticks of grazing cattle in Korea. Vet Parasitol 2012;189:145-152.
11. Masatani T, Yoshihara S, Matsubara A, Gotoh T, Takahashi H, Tanaka T, Andoh M, Endo Y, Matsuo T. Dynamics of Theileria orientalis genotype population in cattle in a year-round grazing system. Acta Parasitol 2016;61:419-424.
12. Sharma A, Singla LD, Ashuma , Batth BK, Kaur P. Clinicopatho-biochemical alterations associated with subclinical babesiosis in dairy animals. J Arthropod Borne Dis 2016;10:258-266.
13. Park J, Han YJ, Han DG, Chae JB, Chae JS, Yu DH, Lee YS, Park BK, Kim HC, Choi KS. Genetic characterization of Theileria orientalis from cattle in the Republic of Korea. Parasitol Res 2017;116:449-454.
14. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4:406-425.
15. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30:2725-2729.
17. Aktas M, Altay K, Dumanli N. A molecular survey of bovine Theileria parasites among apparently healthy cattle and with a note on the distribution of ticks in eastern Turkey. Vet Parasitol 2006;138:179-185.
18. Makala LH, Mangani P, Fujisaki K, Nagasawa H. The current status of major tick borne diseases in Zambia. Vet Res 2003;34:27-45.
20. Yamaguchi T, Yamanaka M, Ikehara S, Kida K, Kuboki N, Mizuno D, Yokoyama N, Narimatsu H, Ikehara Y. Generation of IFN-gamma-producing cells that recognize the major piroplasm surface protein in Theileria orientalis-infected bovines. Vet Parasitol 2010;171:207-215.
21. Gebrekidan H, Gasser RB, Perera PK, McGrath S, McGrath S, Stevenson MA, Jabbar A. Investigating the first outbreak of oriental theileriosis in cattle in South Australia using multiplexed tandem PCR (MT-PCR). Ticks Tick Borne Dis 2015;6:574-578.
22. Perera PK, Gasser RB, Firestone SM, Anderson GA, Malmo J, Davis G, Beggs DS, Jabbar A. Oriental theileriosis in dairy cows causes a significant milk production loss. Parasit Vectors 2014;7:73.
23. Jang DH. Epizootiological study of theileriasis in Korea: Prevalence of the bovine theileriasis in relation to its vector, Haemaphysalis (Kaiseriana) longicornis Neumann. 1901. Korean J Parasitol 1974;12:14-20 (in Korean).
24. Lee MJ, Chae JS. Molecular detection of Ehrlichia chaffeensis and Anaplasma bovis in the salivary glands from Haemaphysalis longicornis ticks. Vector Borne Zoonotic Dis 2010;10:411-413.
25. McFadden AM, Rawdon TG, Meyer J, Makin J, Morley CM, Clough RR, Tham K, Mullner P, Geysen D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N Z Vet J 2011;59:79-85.
26. Chae JS, Heo EJ, Park JH, Choi KS, Dumler JS, Lee SS, Kang TY, Yang JH, Kim DY, Kim JG, Choi GC, Kang MI. Detection of antibodies reacting with Anaplasma phagocytophilum and Ehrlichia chaffeensis from cats, horses and cattle in Korea. J Vet Clin 2009;26:515-519.
27. Hofmann-Lehmann R, Meli ML, Dreher UM, Gonczi E, Deplazes P, Braun U, Engels M, Schupbach J, Jorger K, Thoma R, Griot C, Stark KD, Willi B, Schmidt J, Kocan KM, Lutz H. Concurrent infections with vector-borne pathogens associated with fatal hemolytic anemia in a cattle herd in Switzerland. J Clin Microbiol 2004;42:3775-3780.
28. Shiono H, Yagi Y, Chikayama Y, Miyazaki S, Nakamura I. The influence of oxidative bursts of phagocytes on red blood cell oxidation in anemic cattle infected with Theileria sergenti
. Free Radic Res 2003;37:1181-1189.
29. Terada Y, Ishida M, Yamanaka H. Resistibility to Theileria sergenti infection in Holstein and Japanese Black cattle. J Vet Med Sci 1995;57:1003-1006.
30. Sivakumar T, Hayashida K, Sugimoto C, Yokoyama N. Evolution and genetic diversity of Theileria
. Infect Genet Evol 2014;27:250-263.
31. Hagiwara K, Tokuda M, Baba T, Yamanaka H, Kirisawa R, Tsuji M, Ishihara C, Iwai H. The role of IFN-gamma in cattle infected with Theirelia sergenti
. Vet Parasitol 2005;127:105-110.
32. Savini G, Onuma M, Scaramozzino P, Kakuda T, Semproni G, Langella V. First report of Theileria sergenti and T. buffeli/orientalis in cattle in Italy. Ann N Y Acad Sci 1998;849:404-407.
Fig. 1Molecular detection of Theileria, Anaplasma, and Rickettsia in Hanwoo cattle. PCR was performed to detect (A) 18S rRNA gene of Theileria, (B) 16S rRNA genes of Anaplasma, and (C) Rickettsia in blood samples from Hanwoo cattle. (D) MPSP-based PCR amplification was performed to detect the MPSP gene of T. orientalis in samples positive for the 18S gene of Theileria. Representative images for each pathogen are shown. M, 100 bp DNA ladder. 
Fig. 2Regional infection with tick-borne pathogens in Hanwoo cattle in Korea. Before grazing in spring, infection with tick-borne pathogens was investigated in Hanwoo cattle from 2014 to 2015 in 3 farms; Hoengseong, Jeongeup, and Jeju Island. 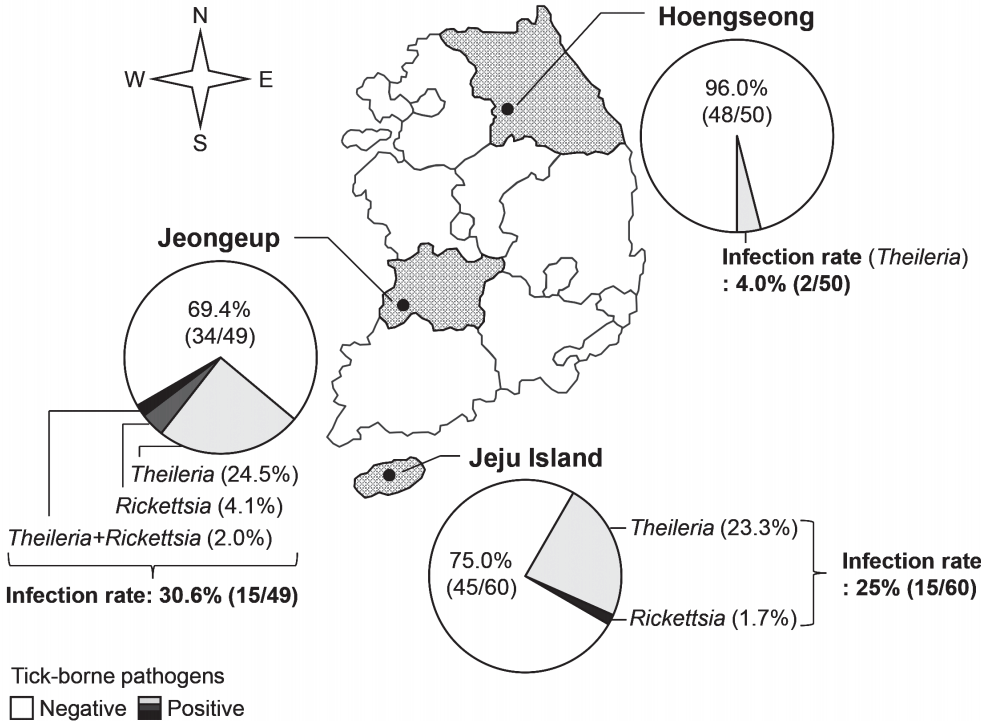
Fig. 3The rate of infection with tick-borne pathogens in grazing Hanwoo cattle. Hanwoo cattle were either housed indoors or maintained outside on the pasture from spring to fall. The rate of infection with tick-borne pathogens was subsequently investigated among (A) housed and (B) pastured cattle. In addition, the infection rate of each pathogen was analyzed in cattle that were positive for tick-borne pathogens. 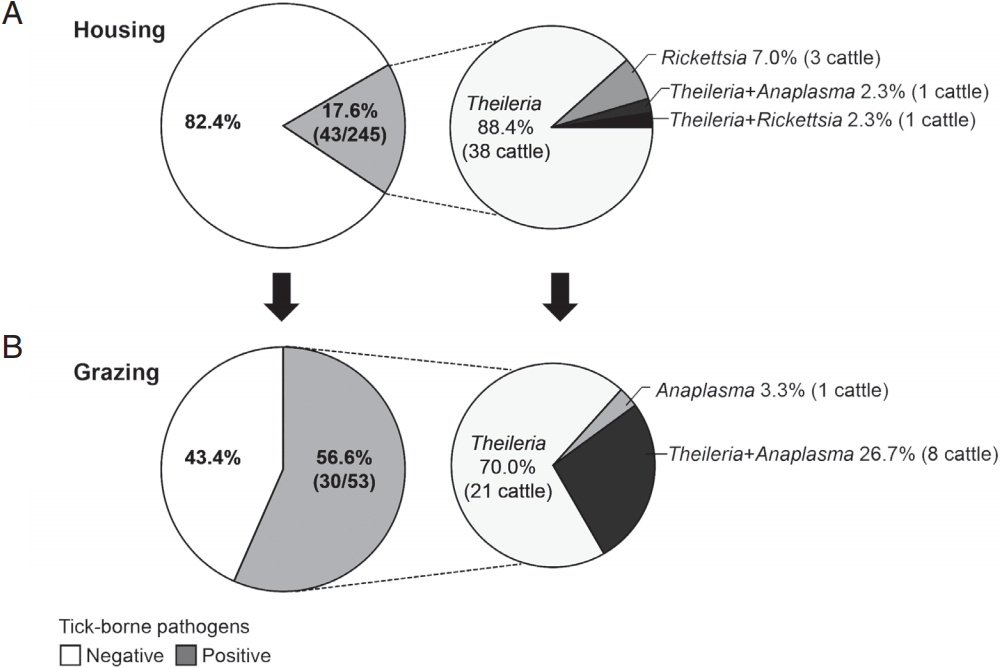
Fig. 4
Theileria orientalis infection and hematological profiles by season and grazing type. (A) The rate of infection with T. orientalis and (B) RBC and HCT values were compared between the housed and grazing cattle during each season. The total indicates the whole season from spring to fall. *P<0.05 and **P<0.01 vs housing within the same season. 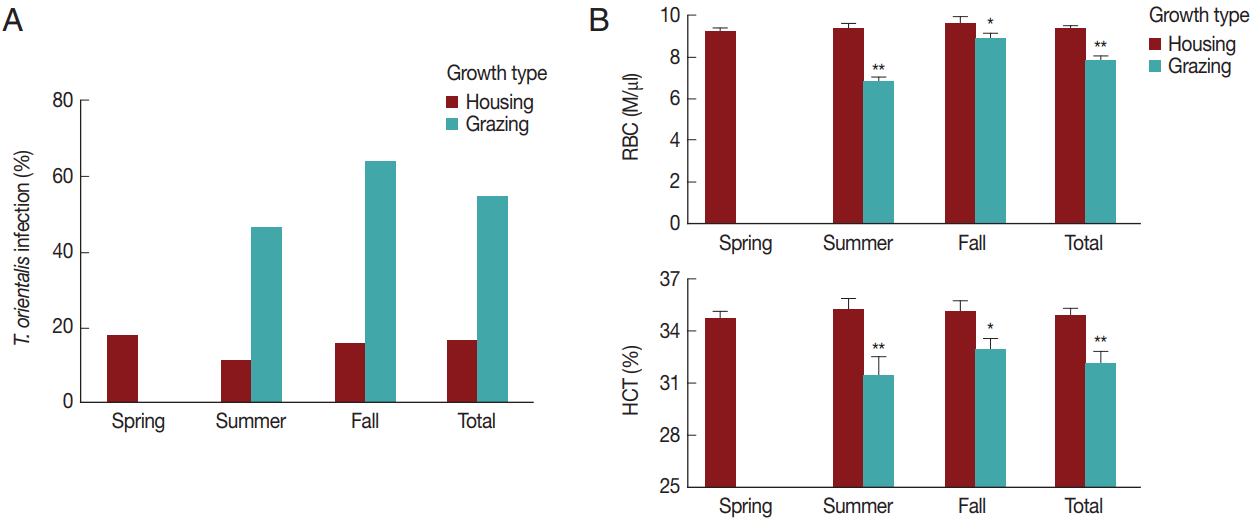
Fig. 5Hematological alterations by Theileria orientalis infection. (A) RBC, (B) Hb, and (C) HCT values were compared between T. orientalis-negative and T. orientalis-positive samples to verify whether T. orientalis induces hematological alterations. N, T. orientalis-negative group (n=221); P, T. orientalis-positive group (n=68). **P<0.01 vs T. orientalis negative group. 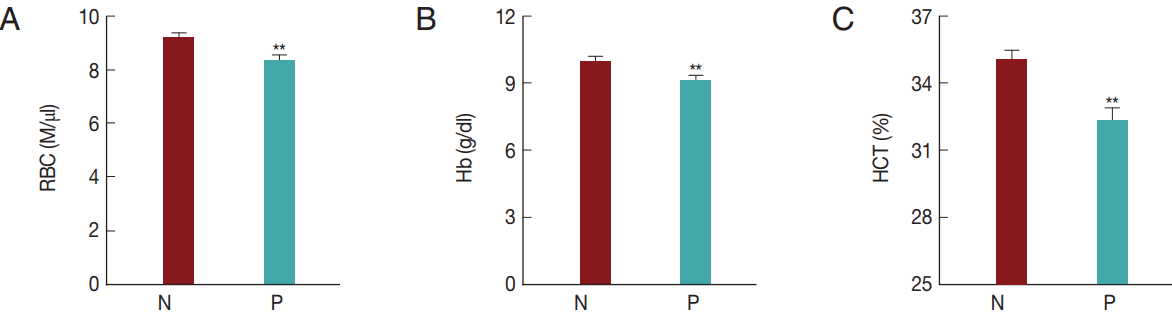
Table 1Hematological profiles and Theileria orientalis in terms of the season and grazing type
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||