INTRODUCTION
Feline hemotropic mycoplasmosis (hemoplasmosis) is primarily associated with 3 hemotropic Mycoplasma species that attach themselves to the surface of red blood cells (RBCs) in cats: Mycoplasma haemofelis (Mhf), Candidatus Mycoplasma haemominutum (CMhm), and Candidatus Mycoplasma turicensis (CMt) [1–6]. Molecular studies have demonstrated the existence of Mhf and CMhm species since 2007 and CMt species since 2017 in feral cats in Korea [7–9]. These species are mainly transmitted through arthropod vectors, such as fleas and ticks, and cat bites, or via blood transfusions [1,2,10–12]. The pathogenicity varies among species, among which Mhf is the most pathogenic [1,2,13,14]. Although the other 2 feline hemoplasma species are not the primary cause of hemolytic anemia, concurrent disease or immune suppression may predispose a cat to life-threatening anemia in these cases [2,15]. One previous case report described a clinical case of Mhf in a cat in Korea; however, clinical reports of other species of Mycoplasma associated with clinical signs have not been reported to date [16]. Here we report clinical cases of CMhm and CMt infections in 2 cats with anemia.
CASE DESCRIPTION
Case 1
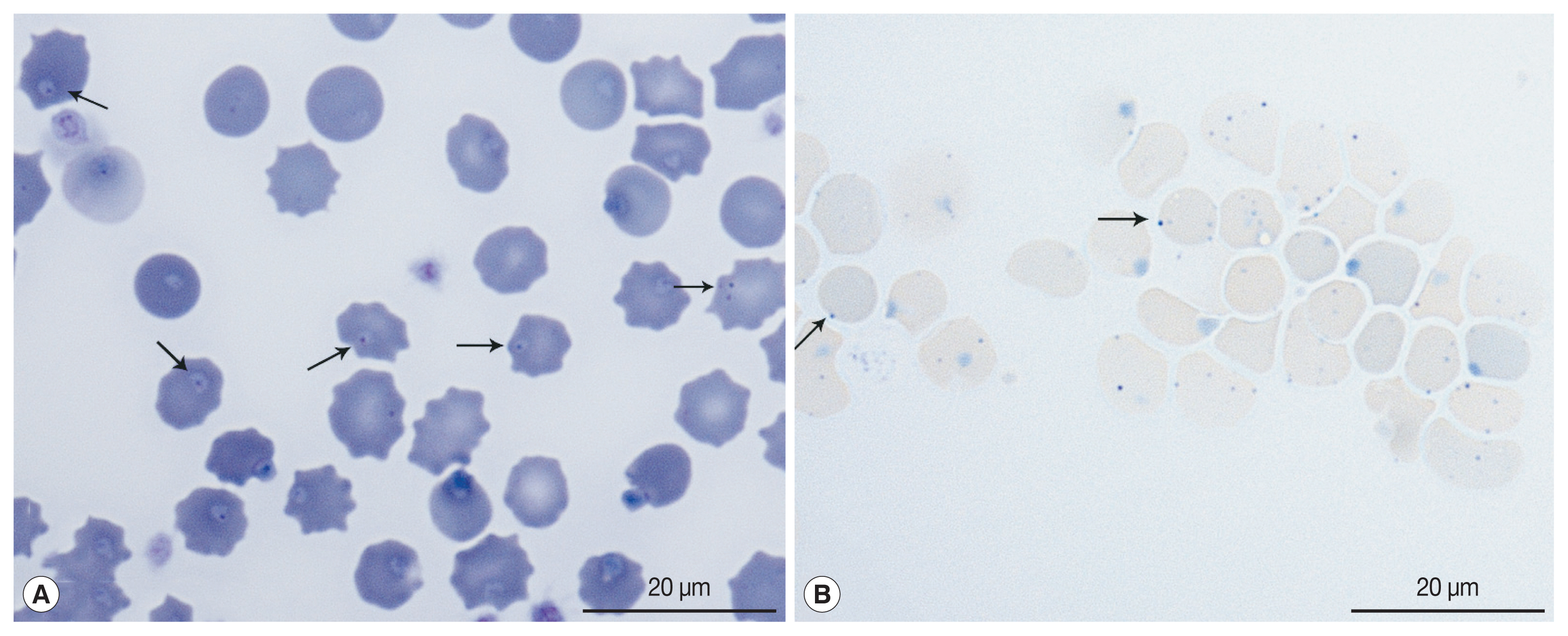
A 6-year-old, client-owned, intact female domestic shorthair cat was admitted to our veterinary medical teaching hospital for an abdominal mass accompanied by peritoneal effusion. The cat was a rescued feral cat, but clinical signs were absent prior to the present illness. Local veterinarians suspected the abdominal mass to be a tumor, and an immunosuppressive dose of prednisolone (2 mg/kg per oral, twice in a day) was administered for 4 weeks. On presentation, the cat had a fever (40.8°C), dehydration, and pale mucous membranes. Manual packed cell measured by microhematocrit tube centrifugation was 25% with a total solid volume of 7.3 mg/dl. A complete blood cell count revealed a mild normocytic normochromic non-regenerative anemia with a red blood cell (RBC) count of 5.36×106/μl (6.54–12.2×106/μl); hematocrit, 23.7% (30.3–52.3%); hemoglobin concentration, 7.9 g/dl (9.8–16.2 g/dl); reticulocyte count, 43.4×103/μl, with leukocytosis 22.71×103/μl (2.87–17.02×103/μl), and a platelet count, 882×103/μl (151–600×103/μl). A blood smear examination stained with Diff-Quik revealed intracellular organisms on the surface of the RBCs (Fig. 1). Molecular analysis to detect feline hemotropic parasites was performed using EDTA anti-coagulated whole blood. Multiplex polymerase chain reaction (PCR) analysis (feline anemia panel, IDEXX laboratory, Westbrook, Maine, USA) showed that the cat was negative for Anaplasma spp., Bartonella spp., Cytauxzoon felis, and Ehrlichia spp., except for feline hemotropic Mycoplasma. Feline leukemia virus and feline immunodeficiency virus infections were excluded using the rapid diagnostic heartworm-feline leukemia virus antigen-feline immunodeficiency virus antibody test kit (IDEXX Laboratories). Abdominal computed tomography revealed asymmetrical wall thickening of the proximal jejunum with loss of the wall layer and heterogeneous contrast enhancement. A round, peripheral enhancing mass was also identified in the abdominal wall adjacent to the 13th chondrocostal junction. Enlarged pancreaticoduodenal and hepatic lymph nodes were revealed. A mass from the abdominal wall revealed a homogenous population of large lymphocytes, with features typical of feline high-grade alimentary lymphoma based on the results of fine-needle aspiration and intestinal mass cytology (Supplementary Fig. S1). A septic exudate due to intestinal perforation was also observed, and the owner elected humane euthanasia. No necropsy was performed per the owner’s preference.
Case 2
An unconscious feral cat with severe dehydration and fever (39.6°C) was rescued. Physical examination revealed tachycardia (240/min), tachypnea (96/min), pale mucous membranes, temporary jerk nystagmus, bilateral purulent oculonasal discharge, and gingivitis with severe dental tartar. Fluid was administered intravenously for resuscitation, and enteral feeding via a nasoesophageal tube was performed for 5 days. Despite doxycycline administration (5 mg/kg, bid), the initial manual packed cell volume of 27% decreased to 16% on day 5. A complete blood count on admission revealed a mild normocytic normochromic non-regenerative anemia, with an RBC count, 5.57×106/μl (6.54–12.2×106/μl); hematocrit, 26.1% (30.3–52.3%); hemoglobin level, 8.3 g/dl (9.8–16.2 g/dl), and reticulocyte count, 12.8×103/μl, along with leukocytosis, 23.85×103/μl (2.87–17.02×103/μl). Feline leukemia virus and feline immunodeficiency virus infections were excluded using the SNAP® Feline Triple® test kit. Molecular analysis was performed to identify the cause of anemia using EDTA anti-coagulated whole blood. Multiplex PCR analysis (feline anemia panel, IDEXX laboratory) revealed that the cat was negative for Anaplasma spp., Bartonella spp., Cytauxzoon felis, and Ehrlichia spp., except for feline hemotropic Mycoplasma. The neurological signs worsened with seizures and nystagmus; euthanasia was performed. The Rivalta test was positive, and necropsy revealed a small amount of viscous and straw-colored pleural/peritoneal effusion. Multifocal granulomatous lesions were observed in the kidneys, mesentery, liver, and brain. Immunohistochemistry with mouse anti-FIP virus monoclonal antibody (Custom Monoclonals International, clone FIPV3-70, Sacramento, California, USA) indicated a feline coronavirus infection in the kidneys and brain, which supported feline infectious peritonitis diagnosis (Supplementary Fig. S2).
Molecular identification of feline hemotropic organisms
The cat’s DNA was extracted from EDTA-anticoagulated whole blood samples using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). Nested PCR targeting 16S rRNA gene was performed to detect feline hemotropic organisms, as described in a previous study [5]. PCR products were visualized by electrophoresis on a 2% agarose gel with ethidium bromide staining. Case 1 had CMhm infection and case 2 had both CMhm and CMt infections. Amplicons were cloned using the TA-cloning Vector Kit II (iNtRON, Seongnam, Korea) to identify the gene sequences. Plasmid DNA for sequencing was purified using the Plasmid Extraction Mini Kit (Favorgen Biotech Corp., PingTung, Taiwan), according to the manufacturer’s instructions. Purified recombinant plasmid DNA was sequenced by 3730 capillary DNA Analyzer (Applied Biosystems, Foster City, California, USA). The DNA sequences were evaluated using Chromas software (Ver 2.6.6), aligned using Clustal X (Ver 2.1), and then examined with a similarity matrix. Phylogenetic trees were created using MEGA 7 [17,18].
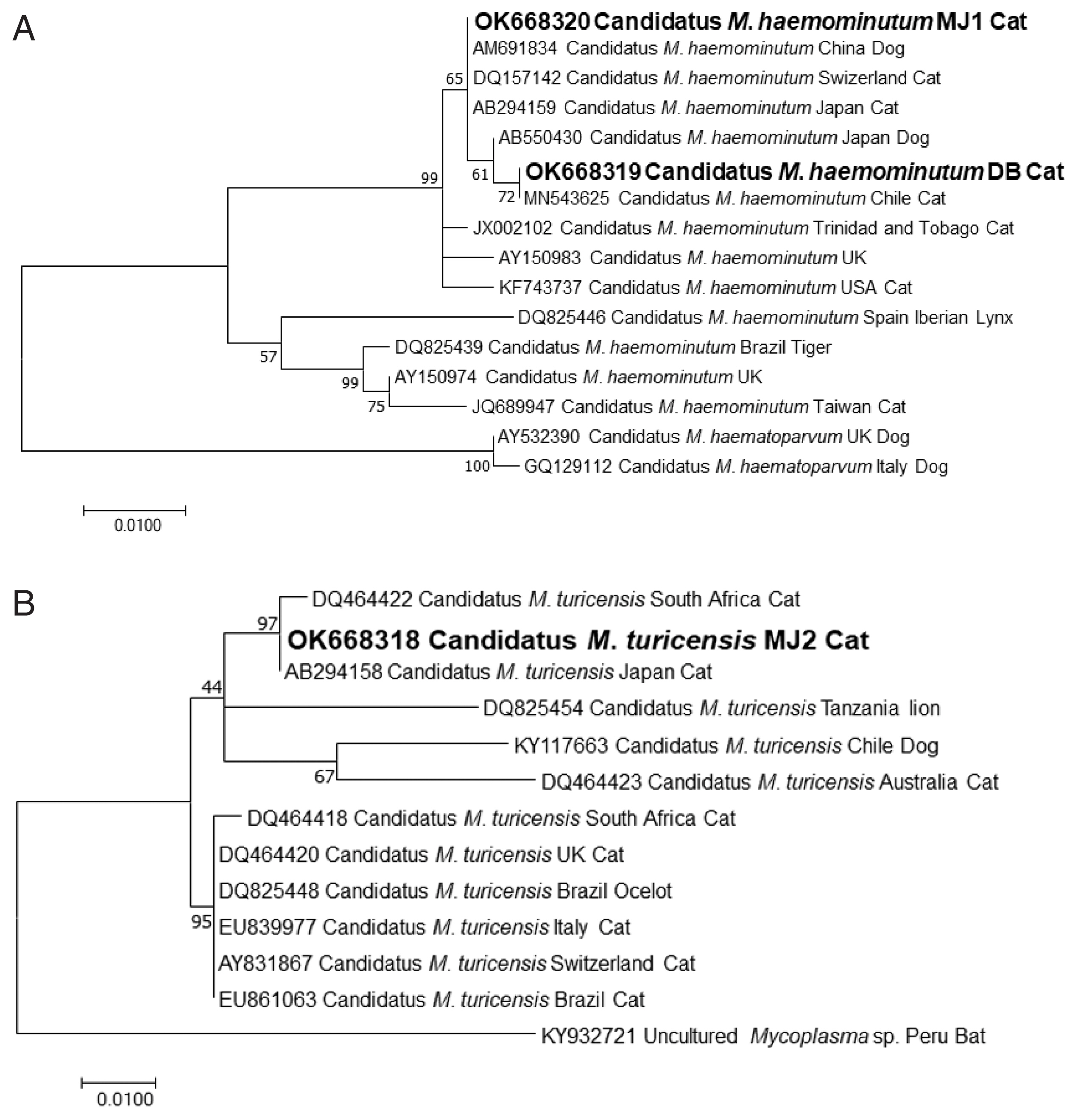
The partial sequences of CMhm from case 1 and CMhm and CMt from case 2 were deposited in the GenBank database with the access no. OK668319, OK668320, and OK668318, respectively. The phylogenetic tree of CMhm 16S rRNA gene is presented in Fig. 2. The partial sequences of the 16S rRNA gene of CMhm and CMt showed 99–100% identity with those from the published sequences from different geographic origins (Fig 2).
The case 1 was diagnosed with subclinical CMhm infection with the clinical signs presenting after the immunosuppressive course of prednisolone, and case 2 was diagnosed with dual infections of CMhm and CMt, causing feline infectious peritonitis.
DISCUSSION
CMt infection was first reported in Switzerland in 2005 [19]. CMhm has the highest prevalence worldwide and the prevalence of Mhf and CMt is similar [1–3,5,6]; the prevalence of CMhm, Mhf, and CMt is 8.5–46.7%, 0.5–21.3%, and 1–10%, respectively [2,3,6]. Although CMhm is known to be more common than Mhf, no studies in Korea have compared the prevalence of these 3 species. Overall, the prevalence of CMhm and Mhf infections is 15.7–19.6% and 4.3–9.6%, respectively [7,8]. It was unknown whether CMt existed in Korea, but a recent study reported a 1.7% prevalence of CMt in feral cats from urban and rural areas [9]. In this study, the existence of CMhm and CMt was confirmed in rescued feral cats. Phylogenetic analysis showed that the partial sequences of CMhm were similar to those from China and Japan. The partial sequences of CMt were 100% identical to those found in Japan. Although no evidence of feline hemoplasmosis among house cats in Korea to date, feline hemoplasmosis should be one of the differential diagnoses if anemic cats are in contact with feral or rescued cats in Korea.
Feline hemoplasma species that are currently recognized show differences in their pathogenicity. A previous Korean article reported Mhf in a 6-month-old rescue cat with severe anemia (hematocrit equal to or less than 15.7%) and jaundice (2.7 mg/dl of total bilirubin) [16]. On the other hand, CMhm and CMt are known to have low pathogenicity, thus do not cause clinically significant signs [14,20]. However, anemia was observed in the present cases, probably due to immunosuppression or preexisting disease [1,2,15,21]. In case 1, an immunosuppressive dose of prednisolone had been administered for 4 weeks before the onset of anemia, and in case 2, necropsy revealed concurrent infection with feline infectious peritonitis. Similar to case 1, a previous study revealed persistent anemia in a cat that worsened after chemotherapy in a cat with lymphoma [21]. Feline infectious peritonitis caused by feline coronaviruses is characterized by fibrinous serositis with fluids accumulation in body cavities, widespread pyogranulomatous lesions, and infection of macrophages and monocytes. Feline infectious peritonitis of viral origin has been associated with severe suppression of natural killer cells and regulatory T cells, leading to cell depletion and decreased cell functionality, whch results in immune suppression [22]. For these reasons, it is believed that both cats in this report were infected with CMhm and CMt, which are less pathogenic, but exhibited clinically significant anemia. To the best of our knowledge, this is the first clinical case report in Korea to demonstrate CMhm and CMt infections with clinical signs in a cat. If an anemic cat has immunosuppression or concurrent disease, feline hemoplasmosis should be included in the differential diagnoses, and PCR should be considered.
Cats with outdoor access are more likely to be infected with hemoplasmas [1,2,10,11], supporting the hypothesis that these agents are indirectly transmitted by blood-sucking arthropods, such as ticks and fleas. Both cats in this report were feral, suggesting that they were more likely to be exposed to these vectors or even involved in fights with other cats, increasing their risk of wounds and infections. Therefore, PCR should be performed if a rescued or feral cat shows symptoms of anemia, and prophylactic antibiotic (doxycycline) administration should be considered because feral cats can be asymptomatically infected with mycoplasma bacteria. Events such as immune suppression may precipitate the onset of anemia.