AbstractLeishmaniasis is a serious parasitic disease caused by Leishmania spp. transmitted through sandfly bites. This disease is a major public health concern worldwide. It can occur in 3 different clinical forms: cutaneous, mucocutaneous, and visceral leishmaniasis (CL, MCL, and VL, respectively), caused by different Leishmania spp. Currently, licensed vaccines are unavailable for the treatment of human leishmaniasis. The treatment and prevention of this disease rely mainly on chemotherapeutics, which are highly toxic and have an increasing resistance problem. The development of a safe, effective, and affordable vaccine for all forms of vector-borne disease is urgently needed to block transmission of the parasite between the host and vector. Immunological mechanisms in the pathogenesis of leishmaniasis are complex. IL-12-driven Th1-type immune response plays a crucial role in host protection. The essential purpose of vaccination is to establish a protective immune response. To date, numerous vaccine studies have been conducted using live/attenuated/killed parasites, fractionated parasites, subunits, recombinant or DNA technology, delivery systems, and chimeric peptides. Most of these studies were limited to animals. In addition, standardization has not been achieved in these studies due to the differences in the virulence dynamics of the Leishmania spp. and the feasibility of the adjuvants. More studies are needed to develop a safe and effective vaccine, which is the most promising approach against Leishmania infection.
INTRODUCTIONLeishmaniasis is a serious parasitic disease caused by Leishmania species transmitted by sand fly bites [1]. The clinical manifestations can be classified as cutaneous (CL), mucocutaneous (MCL), and visceral leishmaniasis (VL). The clinical form depends on the complex interactions between the virulence characteristics of the infecting Leishmania spp. and the immune responses of the host [2]. This disease is a major public health concern. The CL form tends to heal spontaneously and often causes disfiguring scars on the skin [3]. Although a history of CL is not reported in all new MCL patients, MCL results from the spread of parasites to the oral, nasal, pharyngeal, and laryngeal mucosa following or simultaneously with CL. Spontaneous recovery from MCL is rare and can progress to severe deformities such as nasal septal destruction, airway obstruction, and eventually death [4]. The VL is the most severe form and potentially fatal if left untreated [5]. The disease has been reported as endemic in 98 countries and territories in 2020. It primarily affects poor people in Africa, Asia, and Latin America [6]. Despite being the second most common parasitic disease after malaria and its devastating nature, it has remained one of the most neglected diseases worldwide until recent years, perhaps due to its rarity in developed countries [7,8].
Currently, licensed vaccines are unavailable for the treatment of human leishmaniasis [9]. The treatment of the disease and prevention of its spread in the community is mainly dependent on chemotherapy [10–12]. Of the more than 25 compounds, pentavalent antimonials (Sb5+) are the first-line chemotherapy drugs. Amphotericin B, pentamidine isethionate, miltefosine, and paromomycin are the second-line chemotherapeutics [1,13]. However, the existing drugs are not ideal because of their high toxicity and resistance [2,12]. Although the CL form can be treated with thermotherapy, it is not feasible for the other forms of the disease [14].
According to the World Health Organization (WHO), vaccination is most likely the best method to control the disease and avoid the unwanted effects of chemotherapies [14]. The development of a safe, effective, and affordable vaccine for all forms of the disease is one of the most promising approaches and the main priority of global public health [15]. Hence, this article aims to focus on the current status and promising results of the available vaccines evaluated in different modalities.
THE ROLE OF IMMUNOLOGICAL MECHANISMS IN LEISHMANIASISUnlike most parasitic infections, protective immunity occurs against reinfection in patients who recover from leishmaniasis or following leishmanization (LZ) [16,17]. This result reveals that immunological mechanisms play a major role in shaping the disease, and this globally important disease can potentially be prevented using vaccines [8,18,19]. It also provides a roadmap for the development of successful vaccines that can generate protective immunity against infection [20].
The immunological protective mechanism is complicated in leishmaniasis [21]. Cell-mediated immunity plays a crucial role in host immunity. While the primary immune response to infection is initiated through the innate branch, which develops protective innate and adaptive immunity, infection control is mainly mediated by IL-12-driven Th1-type immune response. T lymphocytes shape the host immune response to provide direct protective or non-protective immunity [22]. The production of IFN-γ by CD4+ T cells activates macrophages to kill parasites under nitric oxide (NO)-mediated conditions [23]. Disease progression is largely driven by the production of non-protective IL-4-driven Th2-associated cytokines IL-4, IL-10, IL-13, and TGF-β [24–26]. Some species, such as L. mexicana and L. amazonensis in the New World, unlike L. major, can survive in conditions of limited Th1 immune responses in the host [27]. Unlike Th1, the Th2-type immune response is unable to neutralize intracellular parasites, causing the parasite to spread into VL or, for New World species, disseminated cutaneous leishmaniasis (DCL) [28].
VACCINATION STRATEGIES IN LEISHMANIASISThe availability of vaccines against one or more forms of leishmaniasis can reduce mortality and morbidity associated with this disease. However, Leishmania parasites have a complex life cycle, which consists of stages in animal/human and sand flies and is the most important barrier for vaccine development. Moreover, this neglected tropical disease substantially affects low- and lower-middle-income countries, which discourages commercial developers from invest in the studies of vaccine [8]. In addition, differences in the virulence dynamics of Leishmania species and the immune responses induced by them, as well as the suitability of adjuvants negatively affect vaccine development and standardization efforts [7]. A vaccine against CL caused by L. major may not necessarily have effectivity against the New World forms of diseases, including MCL and DCL [29]. Nonetheless, various vaccination strategies, such as recombinant antigens, DNA vaccines, salivary gland proteins, killed parasites, and live attenuated parasites exist to treat leishmaniasis [30–34].
An ideal antileishmanial vaccine may solve the current problems and must be safe, stable, reproducible, less risky, easily administered, stored, and delivered, not reversible to an infectious state, and able to induce long-term immunological memory [8,35]. However, no vaccine meets these ideal specifications [36]. Compared with other approaches, the strategy of using live vaccines is more attractive because of the induction of a response similar to the immunological response in the natural course of infection (Fig. 1). In the live vaccine strategy, the entire spectrum of antigens is presented to the host immune system without an adjuvant [21].
Potential interventions to induce an immune response against Leishmania can be analyzed using different strategies, such as LZ and saliva vaccines, as well as first-, second-, and third-generation vaccines [28]. Different novel approaches have been investigated for this purpose, such as delivery systems and chimeric peptides. These approaches can be designed as single or cocktail antigens [36–38].
Leishmanization (LZ) vaccineLZ is an ancient practice of vaccination [28]. LZ is an intradermal low-dose inoculation of live and virulent L. major leading to a single lesion [39]. LZ provided greater than 90% protection against reinfection [16]. LZ has been used in several countries in the Middle East and the former Soviet Union. Except for Uzbekistan, which is an endemic country, it is no longer practiced due to safety concerns such as HIV spread, use of immunosuppressive drugs, ethical reasons, uncontrollable persistent skin lesions, and persistence of parasites [16,21]. The overall strategy should be to develop a safer vaccine by providing protective immunity without causing skin lesions, including in immunosuppressed individuals. However, vaccination with live parasites showed a stronger Th1 type of immune response than vaccination with killed parasites, which exhibited limited protection. However, the reason for these differences is not well known [17].
Another study from Iran showed that leishmanization was found to reduce the incidence of the disease between 1/6 and 1/8 of its original level in a hyper-endemic region of Iran and thus was recommended for people at high risk of contracting the disease [39].
Saliva vaccineSand fly vectors (Phlebotomus and Lutzomyia spp.) are of particular interest and induce immune responses as adjuvants through certain immunogenic proteins such as LJM19 and LJL143 from L. longipalpis and PdSP15 from P. duboscqui [16,40,41]. Normally, sand fly saliva is known to enhance the infection caused by Leishmania spp. [41]. However, pre-exposure to saliva protected mice against parasitic infections. In a study on mice, Carregaro et al. [18] reported that the injection of sand fly salivary gland extract resulted in increase in IFN-γ and IL12 production in the site of inflammation and pre-exposure to saliva protects mice against parasitic infections. They concluded that the generation of new saliva vaccine strategies may help prevent Leishmania establishment in the host.
First generation (whole-killed or fractioned parasite) vaccinesFirst-generation vaccines are composed of whole-killed parasites or partially purified fraction(s) or excreted from the parasite. These have been replaced with LZ [28]. The parasites were killed using different methods, such as long-term in vitro culture, temperature, pressure, γ-radiation, and chemicals [44–49]. The killed parasite vaccines present a huge repertoire of parasite antigens and can promote significant protection against infection by mimicking natural infections. However, these studies show that they induce a weaker Th1 type immune response than live parasites, and the results are associated with an inconsistent effect. For these reasons, adjuvants have been used in many studies (e.g., Bacillus Calmette–Guérin (BCG)), and the parasites have been administered through alternative routes such as the mucosal route [19,39, 49].
Whole-killed vaccinesThe Leishvaccine, which comprised whole-killed promastigotes of L. amazonensis strain (IFLA/BR/1967/PH8) and BCG, plays an important role against canine leishmaniasis. The vaccine significantly increases cytokine expression, innate immunity, and adaptive immune response [50]. The leishmania vaccine was successful in clinical trials of human Phases I and II for its safety and immunogenicity; however, it failed in Phase III. The application of autoclaved-killed L. mexicana adjuvanted with BCG resulted in low levels of leishmanin skin test (LST) conversion, which is a marker for cellular immune response [51]. Nevertheless, the incidence of leishmaniasis has significantly decreased in LST-converted participants [49]. Similarly, autoclaved-killed L. major (ALM), the old-world species associated with BCG, caused LST conversion in approximately one-third of healthy participants. However, there was a significant reduction in the incidence in individuals who LST-converted [52].
In addition to their preventive aims, the use of this type of vaccine for immunotherapy offers a safe option for severe forms of CL that do not respond to conventional chemotherapy. In a multicenter randomized controlled clinical trial (RCT) that evaluated the effects of immunotherapy with a vaccine comprising heat-killed L. mexicana+L. amazonensis adjuvanted with BCG over 10 years, 95.7% of patients with CL were treated with mild adverse events and low cost [49]. The immunotherapeutic approach has been successful in cases of mucocutaneous and diffuse forms of CL [52]. Furthermore, a combination of alum-precipitated ALM (alum/ALM)+BCG and sodium stibogluconate (Stb) was shown to be more effective than Stb alone (87% vs. 53%) [53].
Fractionated Leishmania antigensFour fractionated vaccines, Leishmune (Zoetis Industria de Produtos Veterinarios LTDA, São Paulo, Brazil), Leish-Tec (Hertape Calier Saúde Animal S/A, Juatuba, Brazil), CaniLeish (Virbac, Carros, France), and LetiFend (3P Biopharmaceuticals SL, Navarra, Spain), have been licensed and have achieved impressive success in preventing canine leishmaniasis. Of these, Leishmune and Leish-Tec in Brazil, and CaniLeish and LetiFend in Europe have been commercialized [54].
Leishmune is composed of the fucose-mannose ligand (FML) of L. donovani and saponin adjuvant [55]. In phase III studies conducted in dogs, 92% protection against disease was observed. Because no clinical signs or Leishmania DNA were detected in these animals, Leishmune was evaluated as a transmission-blocking vaccine. However, the lack of sample randomization or blinded evaluation of trial individuals did not allow for full validation of the results. In 2014, the production and marketing license of Leishmune was withdrawn [54]. The CaniLeish vaccine is composed of purified excreted–secreted proteins of L. infantum (LiESP) and adjuvanted with saponin (named QA-21) [56]. The vaccine elicited predominantly Th1-type cellular immune responses, and the infection protection rate was 99.4% in a field study [57].
Leish-Tec and LetiFend are recombinant vaccines. Leish-Tec was formulated with recombinant protein A2 from L. donovani amastigotes and saponin as a vaccine adjuvant. In studies that assessed Leish-Tec, it was observed that it was effective not only as preventive, but also in immunotherapeutic approaches [54]. LetiFend contains a chimeric protein (protein Q) formed by 5 antigenic fragments from 4 different L. infantum proteins (ribosomal proteins LiP2a, LiP2b, and LiP0, and histone H2A), to which no adjuvant has been added. It was shown it would be the potential of protein Q in the immunization against L. infantum in preliminary studies in mice [58].
Fractionated Leishmania vaccines seem to be efficiently used in areas that are crucial to the control of Leishmania infection [56].
Second generation (subunit or genetically modified parasite) vaccinesAlthough first-generation vaccines are still being evaluated, several studies have focused on second-generation vaccines. Second-generation vaccines include different recombinant proteins, which are produced through genetically engineered cells such as viruses and bacteria, purified native protein fractions of parasite antigens, synthetic peptides, and even genetically modified parasites [28]. Second-generation vaccines are more feasible for mass vaccination, and their recombinant nature facilitates accessibility to large-scale and cost-effective production [54].
Subunit vaccinesThe several subunits or recombinant vaccine candidates such as LeIF, gp63, p36/LACK, A-2, PSA-2/gp46/M-2, FML, LCR1, ORFF, KMP11, LmSTI1, TSA, HASPB1, protein Q, cysteine protease B (CPB), and A (CPA) have been extensively studied [40,55,59–67]. Many subunit vaccine candidates stimulate an effective protective immune response in the prevention of Leishmania infection. One of the advantages of subunit vaccines is that they pose no risk of infection, which ensures their suitability for immunocompromised individuals [59].
Application of a surface-expressed glycoprotein (gp63), another subunit protein, in a cationic liposome increased the number of IFN-γ-producing effector T cells. Furthermore, vaccination based on gp63 DNA elicited immune responses and conferred protection [60]. In a couple of experiments assessing protective immunological effects, viruses expressing the LACK (Leishmania homologue for receptors of activated C kinase) antigen, with or without adjuvant, showed good protective effects against Leishmania infection [61,62]. Similarly, the virus expressing the promastigote protein surface of G46/M-2/PSA-2 protected against L. amazonensis [63].
Notably, recombinant antigen vaccines such as LEISH-F1, LIESH-F2, and LEISH-F3 have reached phase II clinical trials, demonstrating their potential as vaccine candidates against leishmaniasis [68–70]. LEISH-F1 is an artificial protein encoded by 3 genes: L. major homologue of eukaryotic thiol-specific antioxidant (TSA), L. major stress-inducible protein-1 (LmSTI1), and L. braziliensis elongation and initiation factor (LeIF). LEISH-F1 protein applications were evaluated following emulsification of monophosphoryl lipid A in a structure-stimulating toll-like receptor (MPL-SE). LEISH-F1+MPL-SE (IDRI, Seattle, Washington, USA) efficiently treated patients with CL or ML and induced protective immunity in healthy volunteers [68–70]. This vaccine is also safe and tolerated [71]. Among the other adjuvanted artificial proteins, LEISH-F2+MPL-SE and LEISH-F3+GL-SE (glucopyranosyl lipid A-stable oil-in-water nanoemulsion) showed promising results against infection [68–70]. A NS recombinant vaccine, consisting of enzyme nucleoside hydrolase (NH) and sterol 24-c-methyltransferase (SMT) and adjuvanted with “glucopyranosyl lipid A-stable oil-in-water nanoemulsion” (GLA-SE), is also in clinical trial phase [51].
Genetically modified parasite vaccinesIn Leishmania vaccine research, live attenuated vaccines made by genetic modifications are another research topic [16]. In this strategy, the parasite genes responsible for its survival and/or virulence are modified or deleted. Unlike live virulent parasites, they do not pose any danger associated with infection. However, they ensure that the induction of immune responses is consistent with protection, since they closely mimic natural infection [72].
Various live attenuated vaccine strains of L. major, L. mexicana, L. amazonensis, and L. donovani have been produced by deleting the targeted genes. They were observed to provide significant protection against CL and VL in susceptible mice. Among them, Biopterin transporter 1 (BT1)-deleted L. donovani parasites and A2–rel gene cluster in L. donovani as well as SIR2, Hsp70-II, and KH1 L. infantum null mutants, protected the mice against virulent strains [73–76]. The p27 gene, encoding an amastigote-specific cytochrome C oxidase component, knockout (gene Ldp27−/−) L. donovani reduced parasitic loads and provided long-lasting protection against the development of CL and VL associated with virulent strains [77,78].
Leishmania centrin gene-1 is necessary for parasite growth and differentiation. The generation of a potential mutant vaccine candidate appears to be an interesting target. Volpedo et al. [28] reported that immunization with LdCen−/− led to a significant influx of MHC-II-expressing macrophages, resulting in higher levels of IFN-γ+-secreting CD4+ Th1 cells and lower levels of IL-10- and IL-4-sectreting CD4+ Th2 cells. This response ensures protection against the virulent parasites.
In general, the most promising strategic alternative against VL can be claimed to be the use of live, non-pathogenic/genetically engineered strains of these species [26].
Third generation (naked DNA) vaccinesThird-generation vaccines utilize the DNA [51]. In this relatively new approach, naked plasmid DNA or DNA encapsulated in a viral vector is injected intradermally or intramuscularly [28,79]. DNA vaccines are safe because they do not contain any pathogenic organisms that may revert virulence [79]. They also efficiently induce interferon-gamma production and dendritic cell activation, which protects against Leishmania infection [80].
DNA vaccines can consist of genes encoding single antigens, such as gp63, LACK, or PSA-2. They can also include multiple genes encoding various antigens such as TSA, KMP11, A-2, NH36, LmSTI1, cysteine proteases, and histones [51]. To increase the immunogenicity of DNA vaccines, these vaccines were primed by boosting the associated protein expressed on a recombinant virus-like modified vaccinia virus Ankara [81]. This strategy selectively elicits a wide range of CD8+ T cells specific for Leishmania antigens [28,79].
Collectively, DNA vaccines are stable, do not require adjuvants, produce antigens over long periods, can easily be produced in large quantities, and are effective. Nonetheless, they bear some safety concerns, such as the integration of DNA into the mammalian genome, which can result in the induction of autoimmune diseases or cancer [28].
DNA vaccines, known as third-generation vaccines, are of particular interest because they can effectively induce both CD8+ and CD4+ T cells, produce long-lived antigens and properly folded polypeptides, etc. [82]. Recently, a phase II study is being conducted to evaluate the therapeutic effects of ChAd63-KH, which consists of 2 genes encoding the L. donovani KMP-11 and HASPB antigens, in patients with persistent post-kala-azar dermal leishmaniasis (PKDL) [83].
New strategy vaccinesAdvances in the field of science, in addition to the above-mentioned, have also led to the investigation of new strategies in vaccine development, including certain bioengineering approaches, such as delivery systems and chimeric vaccines, and the use of nonpathogenic Leishmania spp. [19].
Vaccine antigen delivery systemsDrug delivery systems have been extensively studied for the treatment of cancer and infectious diseases [1,88,89]. Nano- particles (NP) are considered ideal vaccine delivery systems. Owing to their structures, vaccine delivery systems present several advantages, such as controlled antigen release, protection of the vaccine antigens from degradation, and site-specific delivery. They can also have adjuvant effect [89]. All these characteristics of NP carriers lead to enhanced bioavailability of antigens, which results in the activation of the immune response. In a study using cationic solid lipid nanoparticles (cSLN) for carrier and delivery, the injection of a fusion gene, pCDNA-A2-CPA-CPB–CTE, enhanced protective cell mediated immunity [90].
Another study demonstrated that vaccination with multifunctionalized PLGA NPs encapsulating sLiAg and/or MPLA provided strong protection against infection with L. infantum [89]. In both studies, the increased production of IFNγ followed by suppression of IL-4 and IL-10 production and very few parasitic loads were considered protective markers. In conclusion, nanoparticle carriers of Leishmania antigen vaccine may be a highly effective strategy against leishmaniasis.
Chimeric vaccinesLage et al. [91] designed an in silico synthetic recombinant vaccine, named ChimeraT. It contained specific T-cell epitopes from Leishmania prohibitin, eukaryotic initiation factor 5a, and hypothetical LiHyp1 and LiHyp2 proteins. After injecting ChimeraT with saponin as an adjuvant, a Th1-type immune response was induced and BALB/c mice were protected against L. infantum infection [91]. In another study, the F1F3 chimera protein (C-terminal domain of nucleoside hydrolase NH36) showed a strong reduction in ear lesion size induced by L. braziliensis. It also promoted the highest CD4+ and CD8+ cytokine-secreting T cell responses, with predominant frequencies of multifunctional CD4+ and CD8+IL-2+TNF-α+IFN-γ+ T cells [92]. Thus, chimeric proteins could be considered potential vaccine candidates to protect against human diseases.
Nonpathogen Leishmania spp. in vaccination
L. tarentolae, isolated from a reptile animal, is a nonpathogenic Leishmania specie in humans [93,94]. In contrast, this parasite activates the dendritic cell maturation process, which results in the production of interferon gamma and induction of a Th1-type immune response. Furthermore, it mimics the natural development of immunity better than other strategies as an important advantage [89]. Breton et al. [95] also observed that intraperitoneally injected L. tarentolae elicited protective immunity against L. donovani in BALB/C mice. In this regard, they proposed that L. tarentolae is a promising live vaccine candidate against Leishmania infections, without causing any infection in humans.
Breton et al. [95] proposed the idea that it can be improved by generating recombinant L. tarentolae expressing selected Leishmania immunodominant epitopes or by combining the L. tarentolae recombinant parasite with a DNA vaccine as part of a prime-boost strategy to elicit more effective and long-lasting protection. In fact, in a parallel study with a cSLN carrier of the pcDNA-A2-CPA-CPB-CTE fusion gene by Taslime et al. [90], it was reported that recombinant L. tarentolae-A2-CPA-CPB-CTE vaccination was protective against L. infantum infection in BALB/c mice.
Viral-like particle vaccinesOne of the latest approaches to developing a safe and cost-effective large-scale vaccine is the use of virus-like particles (VLPs). VLPs are molecules that are morphologically identical to the native virus but cannot replicate because they do not contain viral genetic material (VLPs) [96]. The VLP-based antigen formulation has the potential to generate not only cellular but also humoral immunity that is very similar to that elicited by a natural viral infection VLPs can be produced in different expression systems such as bacterial, yeast, plant, insect or mammalian cells. Panasiuk et al. [86] revealed that VLPs derived from L. tarentolae can induce the production of potent neutralizing antibodies. Maura et al. [97] tested a polyvalent α-Gal, carbohydrates essential for the virulence and viability of many parasites, conjugated to an immunogenic Qβ virus-like particle in a C57BL/6 α-galactosyltransferase knockout mouse model. This vaccine protected knockout mice against L. infantum and L. amazonensis, the aetiological agents of visceral and cutaneous leishmaniasis, respectively.
mRNA vaccinesAn effective vaccine both should boost the natural innate and adaptive immune response and induce a memory immune response that provides long-term protection against infection. Recent research reveals that mRNA vaccines significantly enhance these properties [98]. The mRNA platform aslo allows simultaneous expression of multiple proteins, eliciting immunity against different epitopes from different targets [99]. In a mice model, Duthie et al. [100] observed a significant reduction in the parasite burden in the liver by administering F2-RNA as a prime vaccination and then boosting with the recombinant LEISH-F2 protein. RNA vaccine technology has the potential to offer an effective and practical solution to vaccine development. Development of RNA vaccine requires only knowledge of the target gene sequence, eliminating the need for pathogen culture or scale-up recombinant protein production [101].
CONCLUDING REMARKA safe and efficacious vaccine is urgently required to provide long-lasting protective immunity for the control of parasitic infections. Although cell-mediated immunity is known to play a crucial role in host protection, the immunological protective mechanism of leishmaniasis is complicated. While there is currently no licensed vaccine for human leishmaniasis, extensive efforts are underway to develop a variety of vaccine modalities with promising results worldwide. Some of these modalities are in clinical phase and successful results are obtained in terms of safety and immunogenicity. Meanwhile, it is considered that animal vaccines will play an important role in preventing the transmission of Leishmaniasis to humans. Furthermore, several vaccine candidates are being evaluated at different phases of clinical trials, including those in the first, second, and even third generation.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that may have influenced the work reported in this paper.
REFERENCES1. Dinc R. New developments in the treatment of cutaneous leishmaniasis. Asian Pac J Trop Med 2022;15:196-205 https://doi.org/10.4103/1995-7645.345944
2. Yeşilova Y, Aksoy M, Sürücü HA, Uluat A, Ardic N, Yesilova A. Lip leishmaniasis: Clinical characteristics of 621 patients. Int J Crit Illn Inj Sci 2015;5:265-266 https://doi.org/10.4103/2229-5151.170849
3. Alvar J, den Boer M, Dagne DA. Towards the elimination of visceral leishmaniasis as a public health problem in east Africa: reflections on an enhanced control strategy and a call for action. Lancet Glob Health 2021;9:e1763-1769 https://doi.org/10.1016/S2214-109X(21)00392-2
4. Bezemer JM, Meesters K, Naveda CL, Machado PRL, Calvopiña M, Leeflang MMG, Schallig HDFH, de Vries HJC. Clinical criteria for Mucosal leishmaniasis diagnosis in rural South America: a systematic literature review. PLoS Negl Trop Dis 2022;16:e0010621 https://doi.org/10.1371/journal.pntd.0010621
5. Martins-Melo FR, Lima Mda S, Ramos AN Jr, Alencar CH, Heukelbach J. Mortality and case fatality due to visceral leishmaniasis in Brazil: a nationwide analysis of epidemiology, trends and spatial patterns. PLoS One 2014;9:e93770 https://doi.org/10.1371/journal.pone.0093770
6. World Health Organization. Leishmaniasis: Situation and trend [Internet]; [cited 2022 Aug 29]. Available from: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/leishmaniasis#:~:text=As%20of%20September%202021%2C%2055,Somalia%2C%20South%20Sudan%20and%20Sudan
7. Srivastava S, Shankar P, Mishra J, Singh S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors 2016;9:277 https://doi.org/10.1186/s13071-016-1553-y
8. Malvolti S, Malhame M, Mantel CF, Le Rutte EA, Kaye PM. Human leishmaniasis vaccines: Use cases, target population and potential global demand. PLoS Negl Trop Dis 2021;15:e0009742 https://doi.org/10.1371/journal.pntd.0009742
9. Jha MK, Sarode AY, Bodhale N, Mukherjee D, Pandey SP, Srivastava N, Rub A, Silvestre R, Sarkar A, Saha B. Development and characterization of an avirulent Leishmania major strain. J Immunol 2020;204:2734-2753 https://doi.org/10.4049/jimmunol.1901362
10. Silva CFM, Pinto DCGA, Fernandes PA, Silva AMS. Evolution of acridines and xanthenes as a core structure for the development of antileishmanial agents. Pharmaceuticals (Basel) 2022;15:148 https://doi.org/10.3390/ph15020148
11. Santana W, de Oliveira SS, Ramos MH, Santos ALS, Dolabella SS, Souto EB, Severino P, Jain S. Exploring innovative leishmaniasis treatment: drug targets from pre-clinical to clinical findings. Chem Biodivers 2021;18:e2100336 https://doi.org/10.1002/cbdv.202100336
12. Ardic N, Ardic AF, Gunel Z. Leishmaniasis during the increased Syrian refugee traffic. Glob J Infect Dis Clin Res 2018;4:013-016 http://dx.doi.org/10.17352/2455-5363.000020
13. de Morais RC, de Melo MG, de Goes TC, e Silva RP, de Morais RF, de Oliveira Guerra JA, de Brito ME, Brandão-Filho SP, de Paiva Cavalcanti M. Clinical-therapeutic follow-up of patients with American cutaneous leishmaniasis caused by different Leishmania spp. in Brazil. Exp Parasitol 2022;240:108338 https://doi.org/10.1016/j.exppara.2022.108338
14. World Health Organization. Leishmaniasis[Internet]; [cited 2022 Aug 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
15. Alves F, Bilbe G, Blesson S, Goyal V, Monnerat S, Mowbray C, Muthoni Ouattara G, Pécoul B, Rijal S, Rode J, Solomos A. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev 2018;31:e00048-18 https://doi.org/10.1128/CMR.00048-18
16. Zhang WW, Karmakar S, Gannavaram S, Dey R, Lypaczewski P, Ismail N, Siddiqui A, Simonyan V, Oliveira F, Coutinho-Abreu IV, DeSouza-Vieira T, Meneses C, Oristian J, Serafim TD, Musa A, Nakamura R, Saljoughian N, Volpedo G, Satoskar M, Satoskar S, Dagur PK, McCoy JP, Kamhawi S, Valenzuela JG, Hamano S, Satoskar AR, Matlashewski G, Nakhasi HL. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat Commun 2020;11:3461 https://doi.org/10.1038/s41467-020-17154-z
17. Miramin-Mohammadi A, Javadi A, Eskandari SE, Nateghi-Rostami M, Khamesipour A. Immune responses in cutaneous leishmaniasis: In vitro Thelper1/Thelper2 cytokine profiles using live versus killed Leishmania major. J Arthropod Borne Dis 2021;15:126-135 https://doi.org/10.18502/jad.v15i1.6491
18. Carregaro V, Costa DL, Brodskyn C, Barral AM, Barral-Netto M, Cunha FQ, Silva JS. Dual effect of Lutzomyia longipalpis saliva on Leishmania braziliensis infection is mediated by distinct saliva-induced cellular recruitment into BALB/c mice ear. BMC Microbiol 2013;13:102 https://doi.org/10.1186/1471-2180-13-102
19. Helou DG, Mauras A, Fasquelle F, Lanza JS, Loiseau PM, Betbeder D, Cojean S. Intranasal vaccine from whole Leishmania donovani antigens provides protection and induces specific immune response against visceral leishmaniasis. PLoS Negl Trop Dis 2021;15:e0009627 https://doi.org/10.1371/journal.pntd.0009627
20. Karmakar S, Ismail N, Oliveira F, Oristian J, Zhang WW, Kaviraj S, Singh KP, Mondal A, Das S, Pandey K, Bhattacharya P, Volpedo G, Gannavaram S, Satoskar M, Satoskar S, Sastry RM, Oljuskin T, Sepahpour T, Meneses C, Hamano S, Das P, Matlashewski G, Singh S, Kamhawi S, Dey R, Valenzuela JG, Satoskar A, Nakhasi HL. Preclinical validation of a live attenuated dermotropic Leishmania vaccine against vector transmitted fatal visceral leishmaniasis. Commun Biol 2021;4:929 https://doi.org/10.1038/s42003-021-02446-x
21. Saljoughian N, Taheri T, Rafati S. Live vaccination tactics: possible approaches for controlling visceral leishmaniasis. Front Immunol 2014;5:134 https://doi.org/10.3389/fimmu.2014.00134
22. Nylén S, Maasho K, Söderstrom K, Ilg T, Akuffo H. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon-gamma. Clin Exp Immunol 2003;131:457-467 https://doi.org/10.1046/j.1365-2249.2003.02096.x
23. Kopelyanskiy D, Desponds C, Prevel F, Rossi M, Migliorini R, Snäkä T, Eren RO, Claudinot S, Lye LF, Pasparakis M, Beverley SM, Fasel N. Leishmania guyanensis suppressed inducible nitric oxide synthase provoked by its viral endosymbiont. Front Cell Infect Microbiol 2022;12:944819 https://doi.org/10.3389/fcimb.2022.944819
24. Cecílio P, Cordeiro-da-Silva A, Oliveira F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun Biol 2022;5:305 https://doi.org/10.1038/s42003-022-03240-z
25. Cummings HE, Tuladhar R, Satoskar AR. Cytokines and their STATs in cutaneous and visceral leishmaniasis. J Biomed Biotechnol 2010;2010:294389 https://doi.org/10.1155/2010/294389
26. Ikeogu NM, Akaluka GN, Edechi CA, Salako ES, Onyilagha C, Barazandeh AF, Uzonna JE. Leishmania immunity: advancing immunotherapy and vaccine development. Microorganisms 2020;8:1201 https://doi.org/10.3390/microorganisms8081201
27. Natarajan G, Oghumu S, Varikuti S, Thomas A, Satoskar A. Mechanisms of immunopathology of leishmaniasis. In Satoskar AR, Durvasula R eds, Pathogenesis of Leishmaniasis. Springer. New York, USA. 2014, pp 1-13.
28. Volpedo G, Pacheco-Fernandez T, Bhattacharya P, Oljuskin T, Dey R, Gannavaram S, Satoskar AR, Nakhasi HL. Determinants of innate immunity in visceral leishmaniasis and their implication in vaccine development. Front Immunol 2021;12:748325 https://doi.org/10.3389/fimmu.2021.748325
29. Abdellahi L, Iraji F, Mahmoudabadi A, Hejazi SH. Vaccination in leishmaniasis: a review article. Iran Biomed J 2022;26:1-35 https://doi.org/10.52547/ibj.26.1.35
30. Hosseini Farash BR, Mohebali M, Kazemi B, Fata A, Hajjaran H, Akhoundi B, Raoofian R, Mastroeni P, Moghaddas E, Khaledi A, Salehi Sangani G. Validation of a mixture of rK26 and rK39 antigens from Iranian strain of Leishmania infantum to detect anti-Leishmania antibodies in human and reservoir hosts. Sci Rep 2022;12:10426 https://doi.org/10.1038/s41598-022-14490-6
31. Gomes DC, Souza BL, Schwedersky RP, Covre LP, de Matos Guedes HL, Lopes UG, Ré MI, Rossi-Bergmann B. Intranasal immunization with chitosan microparticles enhances LACK-DNA vaccine protection and induces specific long-lasting immunity against visceral leishmaniasis. Microbes Infect 2022;24:104884 https://doi.org/10.1016/j.micinf.2021.104884
32. Sumova P, Sanjoba C, Willen L, Polanska N, Matsumoto Y, Noiri E, Paul SK, Ozbel Y, Volf P. PpSP32-like protein as a marker of human exposure to Phlebotomus argentipes in Leishmania donovani foci in Bangladesh. Int J Parasitol 2021;51:1059-1068 https://doi.org/10.1016/j.ijpara.2021.05.006
33. Goyal DK, Keshav P, Kaur S. Potential of TLR agonist as an adjuvant in Leishmania vaccine against visceral leishmaniasis in BALB/c mice. Microb Pathog 2021;158:105021 https://doi.org/10.1016/j.micpath.2021.105021
34. Bhaumik SK, Singh MK, Karmakar S, De T. UDP-Gal: N-acetylglucosamine beta 1–4 galactosyltransferase expressing live attenuated parasites as vaccine for visceral leishmaniasis. Glycoconj J 2009;26:663-673 https://doi.org/10.1007/s10719-008-9212-y
35. De Luca PM, Macedo AB. Cutaneous leishmaniasis vaccination: a matter of quality. Front Immunol 2016;7:151 https://doi.org/10.3389/fimmu.2016.00151
36. Pacheco-Fernandez T, Volpedo G, Gannavaram S, Bhattacharya P, Dey R, Satoskar A, Matlashewski G, Nakhasi HL. Revival of leishmanization and leishmanin. Front Cell Infect Microbiol 2021;11:639801 https://doi.org/10.3389/fcimb.2021.639801
37. Ostolin TL, Gusmão MR, Mathias FA, de Oliveira Cardoso JM, Roatt BM, de Oliveira Aguiar-Soares RD, Ruiz JC, de Melo Resende D, de Brito RC, Reis AB. A chimeric vaccine combined with adjuvant system induces immunogenicity and protection against visceral leishmaniasis in BALB/c mice. Vaccine 2021;39:2755-2763 https://doi.org/10.1016/j.vaccine.2021.04.004
38. Athanasiou E, Agallou M, Tastsoglou S, Kammona O, Hatzigeorgiou A, Kiparissides C, Karagouni E. A poly (lactic-co-glycolic) acid nanovaccine based on chimeric peptides from different Leishmania infantum proteins induces dendritic cells maturation and promotes peptide-specific IFNγ-producing CD8+ T cells essential for the protection against experimental visceral leishmaniasis. Front Immunol 2017;8:684 https://doi.org/10.3389/fimmu.2017.00684
39. Mohebali M, Nadim A, Khamesipour A. An overview of leishmanization experience: A successful control measure and a tool to evaluate candidate vaccines. Acta Trop 2019;200:105173 https://doi.org/10.1016/j.actatropica.2019.105173
40. Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine 2013;31:B244-B249 https://doi.org/10.1016/j.vaccine.2012.11.080
41. Oliveira F, Rowton E, Aslan H, Gomes R, Castrovinci PA, Alvarenga PH, Abdeladhim M, Teixeira C, Meneses C, Kleeman LT, Guimarães-Costa AB, Rowland TE, Gilmore D, Doumbia S, Reed SG, Lawyer PG, Andersen JF, Kamhawi S, Valenzuela JG. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med 2015;7:290ra90 https://doi.org/10.1126/scitranslmed.aaa3043
42. Zahedifard F, Gholami E, Taheri T, Taslimi Y, Doustdari F, Seyed N, Torkashvand F, Meneses C, Papadopoulou B, Kamhawi S, Valenzuela JG, Rafati S. Enhanced protective efficacy of nonpathogenic recombinant Leishmania tarentolae expressing cysteine proteinases combined with a sand fly salivary antigen. PLoS Negl Trop Dis 2014;8:e2751 https://doi.org/10.1371/journal.pntd.0009123
43. Davarpanah E, Seyed N, Bahrami F, Rafati S, Safaralizadeh R, Taheri T. Lactococcus lactis expressing sand fly PpSP15 salivary protein confers long-term protection against Leishmania major in BALB/c mice. PLoS Negl Trop Dis 2020;14:e0007939 https://doi.org/10.1371/journal.pntd.0007939
44. Mitchell GF, Handman E, Spithill TW. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust J Exp Biol Med Sci 1984;62:145-153 https://doi.org/10.1038/icb.1984.14
45. Gorczynski RM. Immunization of susceptible BALB/c mice against Leishmania braziliensis,: II. Use of temperature-sensitive avirulent clones of parasite for vaccination purposes. Cellular Immunol 1985;94:11-20 https://doi.org/10.1016/0008-8749(85)90081-4
46. Rivier D, Shah R, Bovay P, Mauel J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol 1993;15:75-84 https://doi.org/10.1111/j.1365-3024.1993.tb00587.x
47. Kimsey PB, Theodos CM, Mitchen TK, Turco SJ, Titus RG. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect Immun 1993;61:5205-5213 https://doi.org/10.1128/iai.61.12.5205-5213.1993
48. Daneshvar H, Coombs GH, Hagan P, Phillips RS. Leishmania mexicana and Leishmania major: attenuation of wild-type parasites and vaccination with the attenuated lines. J Infect Dis 2003;187:1662-1668 https://doi.org/10.1086/374783
49. Convit J, Ulrich M, Polegre MA, Avila A, Rodríguez N, Mazzedo MI, Blanco B. Therapy of Venezuelan patients with severe mucocutaneous or early lesions of diffuse cutaneous leishmaniasis with a vaccine containing pasteurized Leishmania promastigotes and bacillus Calmette-Guerin: preliminary report. Mem Inst Oswaldo Cruz 2004;99:57-62 https://doi.org/10.1590/S0074-02762004000100010
50. Araújo MS, de Andrade RA, Vianna LR, Mayrink W, Reis AB, Sathler-Avelar R, Teixeira-Carvalho A, Andrade MC, Mello MN, Martins-Filho OA. Despite Leishvaccine and Leishmune trigger distinct immune profiles, their ability to activate phagocytes and CD8+ T-cells support their high-quality immunogenic potential against canine visceral leishmaniasis. Vaccine 2008;26:2211-2224 https://doi.org/10.1016/j.vaccine.2008.02.044
51. Moafi M, Rezvan H, Sherkat R, Taleban R. Leishmania vaccines entered in clinical trials: A review of literature. Int J Prev Med 2019;10:95 https://doi.org/10.4103/ijpvm.IJPVM_116_18
52. Convit J, Ulrich M, Zerpa O, Borges R, Aranzazu N, Valera M, Villarroel H, Zapata Z, Tomedes I. Immunotherapy of american cutaneous leishmaniasis in Venezuela during the period 1990–99. Trans R Soc Trop Med Hyg 2003;97:469-472 https://doi.org/10.1016/S0035-9203(03)90093-9
53. Khamesipour A. Therapeutic vaccines for leishmaniasis. Expert Opin Biol Ther 2014;141641-9 https://doi.org/10.1517/14712598.2014.945415
54. Velez R, Gállego M. Commercially approved vaccines for canine leishmaniosis: a review of available data on their safety and efficacy. Trop Med Int Health 2020;25:540-557 https://doi.org/10.1111/tmi.13382
55. Borja-Cabrera GP, Santos FN, Bauer FS, Parra LE, Menz I, Morgado AA, Soares IS, Batista LMM, Palatnik-de-Sousa CB. Immunogenicity assay of the Leishmune® vaccine against canine visceral leishmaniasis in Brazil. Vaccine 2008;26:4991-4997 https://doi.org/10.1016/j.vaccine.2008.07.029
56. The European Public Assessment. CaniLeish: canine vaccine against Leishmania infantum adjuvanted [Internet]. The European Public Assessment; [cited 2022 Aug 29]. Available from: https://www.ema.europa.eu/en/documents/overview/canileish-epar-summary-public_en.pdf
57. Moreno J, Vouldoukis I, Martin V, McGahie D, Cuisinier AM, Gueguen S. Use of a LIESP/QA-21 vaccine (CaniLeish) stimulates an appropriate Th1-dominated cell-mediated immune response in dogs. PLoS Negl Trop Dis 2012;6:e1683 https://doi.org/10.1371/journal.pntd.0001683
58. Parody N, Soto M, Requena JM, Alonso C. Adjuvant guided polarization of the immune humoral response against a protective multicomponent antigenic protein (Q) from Leishmania infantum. A CpG+Q mix protects Balb/c mice from infection. Parasite Immunol 2004;26:283-293 https://doi.org/10.1111/j.0141-9838.2004.00711.x
59. Ahmed SB, Bahloul C, Robbana C, Askri S, Dellagi K. A comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L. major. Vaccine 2004;22:1631-1639 https://doi.org/10.1016/j.vaccine.2003.10.046
60. Olobo JO, Anjili CO, Gicheru MM, Mbati PA, Kariuki TM, Githure JI, Koech DK, McMaster WR. Vaccination of vervet monkeys against cutaneous leishmaniosis using recombinant Leishmania ‘major surface glycoprotein’ (gp63). Vet Parasitol 1995;60:199-212 https://doi.org/10.1016/0304-4017(95)00788-6
61. Gurunathan S, Sacks DL, Brown DR, Reiner SL, Charest H, Glaichenhaus N, Seder RA. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med 1997;186:1137-1147 https://doi.org/10.1084/jem.186.7.1137
62. Yang DM, Fairweather N, Button LL, McMaster WR, Kahl LP, Liew FY. Oral Salmonella typhimurium (AroA-) vaccine expressing a major Leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol 1990;145:2281-2285.
63. McMahon-Pratt D, Rodriguez D, Rodriguez JR, Zhang Y, Manson K, Bergman C, Rivas L, Rodriguez JF, Lohman KL, Ruddle NH. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect Immun 1993;61:3351-3359 https://doi.org/10.1128/iai.61.8.3351-3359.1993
64. Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun 1988;56:3272-3279 https://doi.org/10.1128/iai.56.12.3272-3279.1988
65. Handman E, Symons FM, Baldwin TM, Curtis JM, Scheerlinck JP. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a TH1 type of immune response. Infect Immun 1995;63:4261-4267 https://doi.org/10.1128/iai.63.11.4261-4267.1995
66. Santos WR, Aguiar IA, de Souza EP, de Lima VM, Palatnik M, Palatnik-de-Sousa CB. Immunotherapy against murine experimental visceral leishmaniasis with the FML-vaccine. Vaccine 2003;21:4668-4676 https://doi.org/10.1016/S0264-410X(03)00527-9
67. Nagill R, Kaur S. Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol 2011;11:1464-1488 https://doi.org/10.1016/j.intimp.2011.05.008
68. Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, Rolf T, Misquith A, Laurance JD, Raman VS, Bailor HR, Cauwelaert ND, Reed SJ, Vallur A, Favila M, Orr MT, Ashman J, Ghosh P, Mondal D, Reed SG. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunology 2015;4:e35 https://doi.org/10.1038/cti.2015.6
69. Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 2016;34:2992-2995 https://doi.org/10.1016/j.vaccine.2015.12.071
70. Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, Laughlin EM, Coler RN, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Sundar S, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine 2011;29:3531-3537 https://doi.org/10.1016/j.vaccine.2011.02.096
71. Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine 2012;30:134-141 https://doi.org/10.1016/j.vaccine.2011.11.005
72. Gannavaram S, Dey R, Avishek K, Selvapandiyan A, Salotra P, Nakhasi HL. Biomarkers of safety and immune protection for genetically modified live attenuated leishmania vaccines against visceral leishmaniasis-discovery and implications. Front Immunol 2014;5:241 https://doi.org/10.3389/fimmu.2014.00241
73. Papadopoulou B, Roy G, Breton M, Kündig C, Dumas C, Fillion I, Singh AK, Olivier M, Ouellette M. Reduced infectivity of a Leishmania donovani biopterin transporter genetic mutant and its use as an attenuated strain for vaccination. Infect Immun 2002;70:62-68 https://doi.org/10.1128/IAI.70.1.62-68.2002
74. Silvestre R, Cordeiro-Da-Silva A, Santarém N, Vergnes B, Sereno D, Ouaissi A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. J Immunol 2007;179:3161-3170 https://doi.org/10.4049/jimmunol.179.5.3161
75. Carrión J, Folgueira C, Soto M, Fresno M, Requena JM. Leishmania infantum HSP70-II null mutant as candidate vaccine against leishmaniasis: a preliminary evaluation. Parasit Vectors 2011;4:150 https://doi.org/10.1186/1756-3305-4-150
76. Ghosh A, Labrecque S, Matlashewski G. Protection against Leishmania donovani infection by DNA vaccination: increased DNA vaccination efficiency through inhibiting the cellular p53 response. Vaccine 2001;19:3169-3178 https://doi.org/10.1016/S0264-410X(01)00023-8
77. Dey R, Dagur PK, Selvapandiyan A, McCoy JP, Salotra P, Duncan R, Nakhasi HL. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J Immunol 2013;190:2138-2149 https://doi.org/10.4049/jimmunol.1202801
78. Walker PS, Scharton-Kersten T, Rowton ED, Hengge U, Bouloc A, Udey MC, Vogel JC. Genetic immunization with glycoprotein 63 cDNA results in a helper T cell type 1 immune response and protection in a murine model of leishmaniasis. Hum Gene Ther 1998;9:1899-1907 https://doi.org/10.1089/hum.1998.9.13-1899
79. Mutiso JM, Macharia JC, Kiio MN, Ichagichu JM, Rikoi H, Gicheru MM. Development of Leishmania vaccines: predicting the future from past and present experience. J Biomed Res 2013;27:85-102 https://doi.org/10.7555/JBR.27.20120064
80. Aunguldee T, Gerdprasert O, Tangteerawatana P, Jariyapongskul A, Leelayoova S, Wongsatayanon BT. Immunogenicity and potential protection of DNA vaccine of Leishmania martiniquensis against Leishmania infection in mice. J Infect Dev Ctries 2021;15:1328-1338 https://doi.org/10.3855/jidc.14472
81. Rafati S, Zahedifard F, Azari MK, Taslimi Y, Taheri T. Leishmania infantum: prime boost vaccination with C-terminal extension of cysteine proteinase type I displays both type 1 and 2 immune signatures in BALB/c mice. Exp Parasitol 2008;118:393-401 https://doi.org/10.1016/j.exppara.2007.10.004
82. Prasanna P, Kumar P, Kumar S, Rajana VK, Kant V, Prasad SR, Mohan U, Ravichandiran V, Mandal D. Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis–a review. Biomed Pharmacother 2021;141:111920 https://doi.org/10.1016/j.biopha.2021.111920
83. Kaye P; University of York. A study to assess the safety, efficacy and immunogenicity of Leishmania vaccine ChAd63-KH in PKDL [Internet];; [cited 2022 Dec 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT03969134
84. Taheri T, Rafati S. Leishmaniasis: recombinant DNA vaccination and different approaches for vaccine development. Clin Invest 2013;3:1023-1044 https://doi.org/10.4155/CLI.13.99
85. Volpedo G, Huston RH, Holcomb EA, Pacheco-Fernandez T, Gannavaram S, Bhattacharya P, Nakhasi HL, Satoskar AR. From infection to vaccination: reviewing the global burden, history of vaccine development, and recurring challenges in global leishmaniasis protection. Expert Rev Vaccines 2021;20:1431-1446 https://doi.org/10.1080/14760584.2021.1969231
86. Cecílio P, Pérez-Cabezas B, Fernández L, Moreno J, Carrillo E, Requena JM, Fichera E, Reed SG, Coler RN, Kamhawi S, Oliveira F, Valenzuela JG, Gradoni L, Glueck R, Gupta G, Cordeiro-da-Silva A. Pre-clinical antigenicity studies of an innovative multivalent vaccine for human visceral leishmaniasis. PLoS Negl Trop Dis 2017;11:e0005951 https://doi.org/10.1371/journal.pntd.0005951
87. Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, Wiggins R, Di Marco S, Colloca S, Siani L, Cortese R, Smith DF, Aebischer T, Kaye PM, Lacey CJ. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl Trop Dis 2017;11:e0005527 https://doi.org/10.1371/journal.pntd.0005527
88. Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol 2018;9:2224 https://doi.org/10.3389/fimmu.2018.02224
89. Margaroni M, Agallou M, Athanasiou E, Kammona O, Kiparissides C, Gaitanaki C, Karagouni E. Vaccination with poly(D,L-lactide-co-glycolide) nanoparticles loaded with soluble Leishmania antigens and modified with a TNFα-mimicking peptide or monophosphoryl lipid A confers protection against experimental visceral leishmaniasis. Int J Nanomedicine 2017;12:6169-6184 https://doi.org/10.2147/IJN.S141069
90. Taslimi Y, Zahedifard F, Taheri T, Doroud D, Dizaji SL, Saljoughian N, Rafati S. Comparison of protective potency of DNA and live vaccines expressing A2-CPA-CPB−CTE antigens against visceral leishmaniasis in Syrian hamster as preliminary study. Iran J Parasitol 2020;15:383-392 https://doi.org/10.18502/ijpa.v15i3.4203
91. Lage DP, Ribeiro PA, Dias DS, Mendonça DV, Ramos FF, Carvalho LM, de Oliveira D, Steiner BT, Martins VT, Perin L, Machado AS, Santos TTO, Tavares GSV, Oliveira-da-Silva JA, Oliveira JS, Roatt BM, Machado-de-Ávila RA, Teixeira AL, Humbert MV, Coelho EAF, Christodoulides M. A candidate vaccine for human visceral leishmaniasis based on a specific T cell epitope-containing chimeric protein protects mice Against Leishmania infantum infection. NPJ Vaccines 2020;5:75 https://doi.org/10.1038/s41541-020-00224-0
92. Alves-Silva MV, Nico D, de Luca PM, Palatnik de-Sousa CB. The F1F3 recombinant chimera of Leishmania donovani-Nucleoside Hydrolase (NH36) and its epitopes induce cross-protection against Leishmania (V.) braziliensis infection in mice. Front Immunol 2019;10:724 https://doi.org/10.3389/fimmu.2019.00724
93. Klatt S, Simpson L, Maslov DA, Konthur Z. Leishmania tarentolae: taxonomic classification and its application as a promising biotechnological expression host. PLoS Negl Trop Dis 2019;13:e0007424 https://doi.org/10.1371/journal.pntd.0007424
94. Mendoza-Roldan JA, Latrofa MS, Iatta R, Manoj RSR, Panarese R, Annoscia G, Pombi M, Zatelli A, Beugnet F, Otranto D. Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities. Parasit Vectors 2021;14:461 https://doi.org/10.1186/s13071-021-04973-2
95. Breton M, Tremblay MJ, Ouellette M, Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect Immun 2005;73:6372-6382 https://doi.org/10.1128/IAI.73.10.6372-6382.2005
96. Panasiuk M, Zimmer K, Czarnota A, Grzyb K, Narajczyk M, Peszyńska-Sularz G, Żołędowska S, Nidzworski D, Hovhannisyan L, Gromadzka B. Immunization with Leishmania tarentolae-derived norovirus virus-like particles elicits high humoral response and stimulates the production of neutralizing antibodies. Microbial Cell Factories 2021;20:186 https://doi.org/10.1186/s12934-021-01677-1
97. Moura AP, Santos LC, Brito CR, Valencia E, Junqueira C, Filho AA, Sant’Anna MR, Gontijo NF, Bartholomeu DC, Fujiwara RT, Gazzinelli RT, McKay CS, Sanhueza CA, Finn MG, Marques AF. Virus-like particle display of the α-Gal carbohydrate for vaccination against Leishmania infection. ACS central science 2017;3:1026-1031 https://doi.org/10.1021/acscentsci.7b00311
98. De Beuckelaer A, Grooten J, De Koker S. Type I interferons modulate CD8+ T cell immunity to mRNA vaccines. Trends Mol Med 2017;23:216-226 https://doi.org/10.1016/j.molmed.2017.01.006
99. Versteeg L, Almutairi MM, Hotez PJ, Pollet J. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines 2019;7:122 https://doi.org/10.3390/vaccines7040122
100. Duthie MS, Van Hoeven N, MacMillen Z, Picone A, Mohamath R, Erasmus J, Hsu FC, Stinchcomb DT, Reed SG. Heterologous immunization with defined RNA and subunit vaccines enhances T Cell responses that protect against Leishmania donovani. Front Immunol 2018;9:2420 https://doi.org/10.3389/fimmu.2018.02420
101. Duthie MS, Machado BA, Badaró R, Kaye PM, Reed SG. Leishmaniasis vaccines: applications of RNA technology and targeted clinical trial designs. Pathogens 2022;11:1259 https://doi.org/10.3390/pathogens11111259
Fig. 1Schematic depiction briefing the immunological response against Leishmania infection (Modified from Pacheco-Fernandez et al. [36], with permission). (A) Immunological response in live/attenuated Leishmania vaccination. 1) Injection of live/attenuated parasites, 2) Transforming promastigotes into amastigotes, 3) Internalization of the amastigotes by dendritic cells, 4) Presentation to T cells in the draining lymph nodes by dendritic cells, 5) Differentiation of T cells into effector and memory T cells, 6) Prevention of transmission to the sand fly because of long-term protective immunity. (B) Immunological response in Leishmania vaccination using DNA, recombinant antigen (Ag), or subunit Ag. 1) Injection of DNA, recombinant Ag, or subunit Ag, 2) Encountering of antigens and dendritic cells, 3) Internalization of the antigens by dendritic cells, 4) Presentation to T cells in the draining lymph nodes by dendritic cells, 5) Differentiation T cells into the effector and memory T cells, 6) Prevention of transmission to the sand fly because of long-term protective immunity. CD4+ TEff: effector T helper cell, CD4+ TMem: memory T helper cell, CD4+ TCent Mem: central memory T helper cell, Tissue TRes Mem: tissue residence memory T cell, effector T helper cell, and CD8+ TEff: effector cytotoxic T cell. 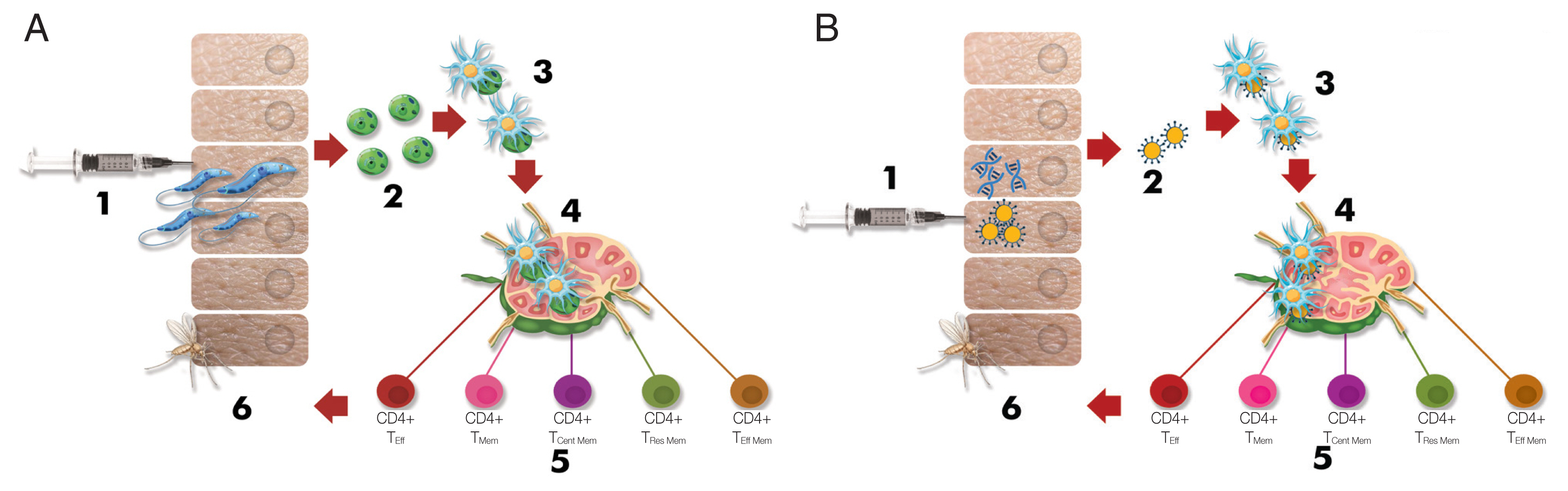
Table 1CL, Cutaneous leishmaniasis; VL, visceral leishmaniasis; MCL, Cutaneous leishmaniasis; PKDL, post-kala-azar dermal leishmaniasis; BCG, Bacillus Calmette Guerin; BT1, Biopterin transporter 1; Ldp27, L. donovani amastigote specific protein p27; Cen, centrin; HSP, Heat-schok protein; A2, Amastigote specific protein 2; LACK, Leishmania homolog of receptors for activated c-kinase; CPA or B, Cysteine peptidase A or B; NH, Nucleoside hydrolase; SMT, Sterol 24-c-methyltransferase; H1, Histone-1; LJM19, A L. longipalpis salivary protein; LJL143, A L. longipalpis salivary protein; PdSP15, P. duboscqi salivary protein-15; KMH-11, Kinetoplastid membrane protein 11; TSA, Thiol-specific antioxidant; gp63, Gikoprotein63; LmSTI1, L. major stress-inducible protein-1; LeIF, L. braziliensis elongation and initiation factor; MIDGE, minimalistic immunogenically defined gene expression. Table 2ALM, Autoclaved-killed L. major; GALM, Gentamycin-attenuated L. major; NH, Nucleoside hydrolase; SMT, Sterol 24-c-methyltransferase; FML, fucose-mannose ligand; LiESP, L. infantum excreted–secreted protein; KMH-11, Kinetoplastid membrane protein 11; HASB, Hydrophilic acylated surface protein B; BCG, Bacillus Calmette Guerin; MPL-SE, Monophosphoryl lipid A; GLA-SE, Glucopyranosyl lipid A-stable oil-in-water nano emulsion. |
|
||||||||||||||||||||||||||||||||||||||||||