INTRODUCTION
The long-tailed goral (Naemorhedus caudatus) is internationally listed as a rare species distributed in the northeast Asia including Russia, China, and the Korean Peninsula. Its global population size is estimated to be 2,500–10,000 for mature individuals, and this number is decreasing [1]. In Korea, approximately 6,000 individuals of long-tailed gorals were trapped by heavy snowfalls in Gangwon-do in 1950s and 1960s, resulting in tremendous decreases in its numbers [2]. A small population was also detected in demilitarized zone (DMZ) and Seoraksan (san=mountain) up to 1980s. On-going forest utilization and habitat fragmentation pose an anthropogenic threats to long-tailed gorals population in Korea. A nationwide investigation in 2002 reported 690–784 individuals. The numbers and distributions of long-tailed goral have started expanding to new regions since 2010 [3].
Recently, 2 long-tailed gorals were rescued from Uljin-gun (gun=county), Gyeongsangbuk-do (do=province) and Cheorwon-gun, Gangwon-do, ca. 200 km apart from each other in Korea. These long-tailed gorals were physically diagnosed with scabies infestation. However, such an infection in this species has not been reported precisely in Korea and other countries. On the other hand, the itch mite (Sarcoptes scabiei) is an ectoparasite known to burrow in the epidermis of the skin of human and other mammals along their specialized propagation life cycle of approximately 2 weeks [4]. Itch mites that burrow into the skin produce inflammatory substances and stimulate the host’s immune responses, resulting in deteriorated host protective mechanisms [4]. With respect to the cross-infectivity of ectoparasite infestation, S. scabiei can be transmitted among domestic dogs, wild canines, rabbits, and diverse ungulates through their social or predator-prey interactions [4,5]. Here, we report 2 cases of mange mite infestation in endangered long-tailed gorals rescued in 2 different regions in Korea by analyzing morphological and phylogenetic characteristics.
CASE DESCRIPTION
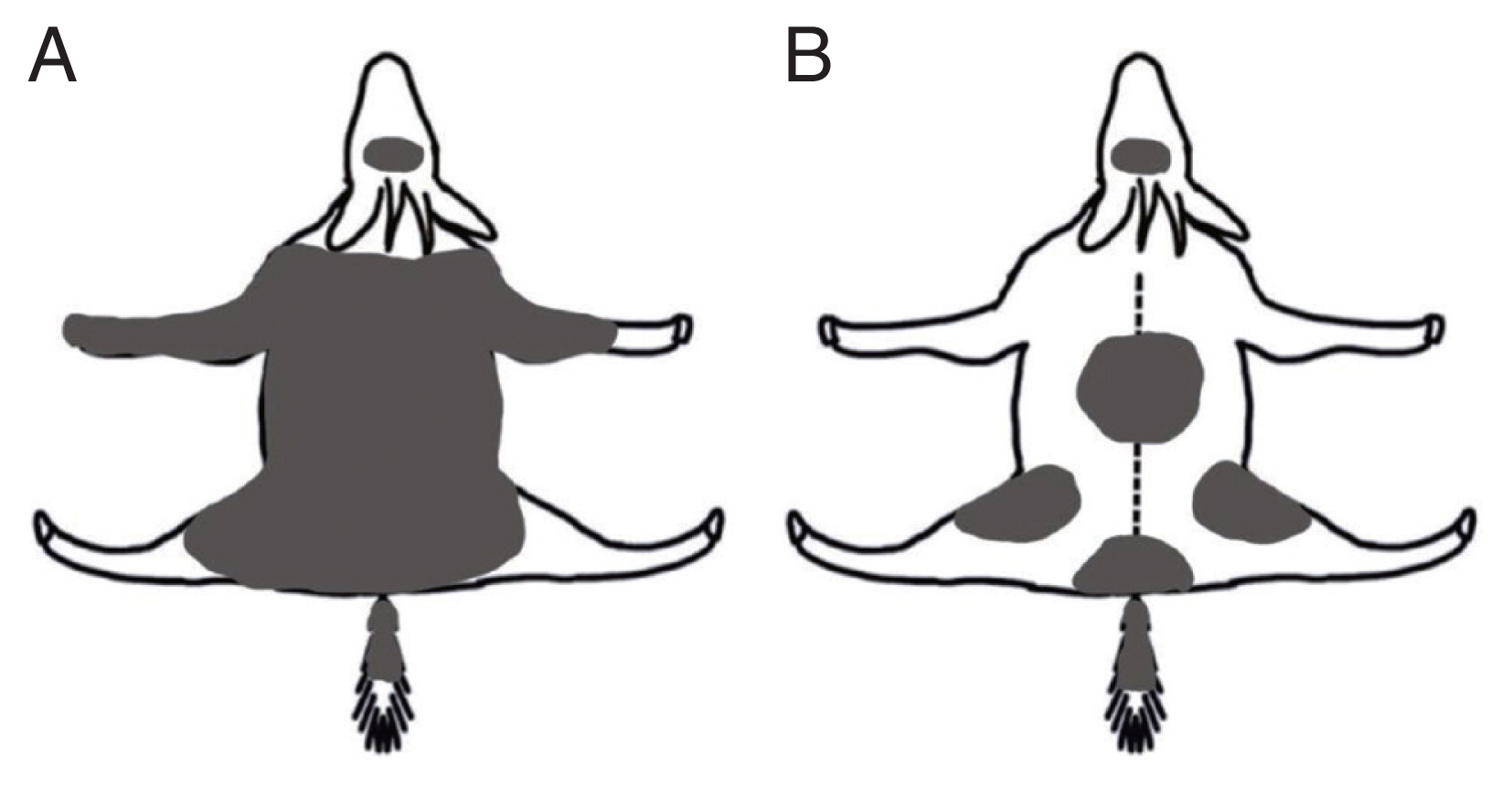
An adult female goral, 7–8 years old, was rescued from a known habitat, Geumgangsong-myeon (myeon=township), in Uljin-gun (36º54′21.29″N, 129º10′33.23″E) in March 25, 2021, but immediately expired. At necropsy, extensive hair loss and epidermal keratosis were found on the skin with sarcoptic manges. The goral exhibited severe starvation and dehydration in the comatose state (weight 29.8 kg). On genital examination, a trace of miscarriage was found. The stomach was filled with grasses (i.e., normal foraging). However, no other fatal conditions were found in necropsy. The other goral, about 2 month old female (5.4 kg weight), was rescued from an unknown habitat, Geunnam-myeon in Cheorwon-gun (38º15′29.03″N, 127º34′57.92″E) by the Korean Association for Bird Protection in August 19, 2021. The next day, the goral was transferred to Research Center for Endangered Species, National Institute of Ecology. The cutaneous keratosis were obviously observed in the skin of limb joints, edges of ears, and tail. We sampled sarcoptic mites from the skin legions and diagnosed them as the scabies. The female goral recovered after diverse veterinary examinations and treatments (e.g., application of ivermectin). Overall, Uljin female exhibited more severe and advanced symptoms of sarcoptic mange than Cheorwon one (Fig. 1).
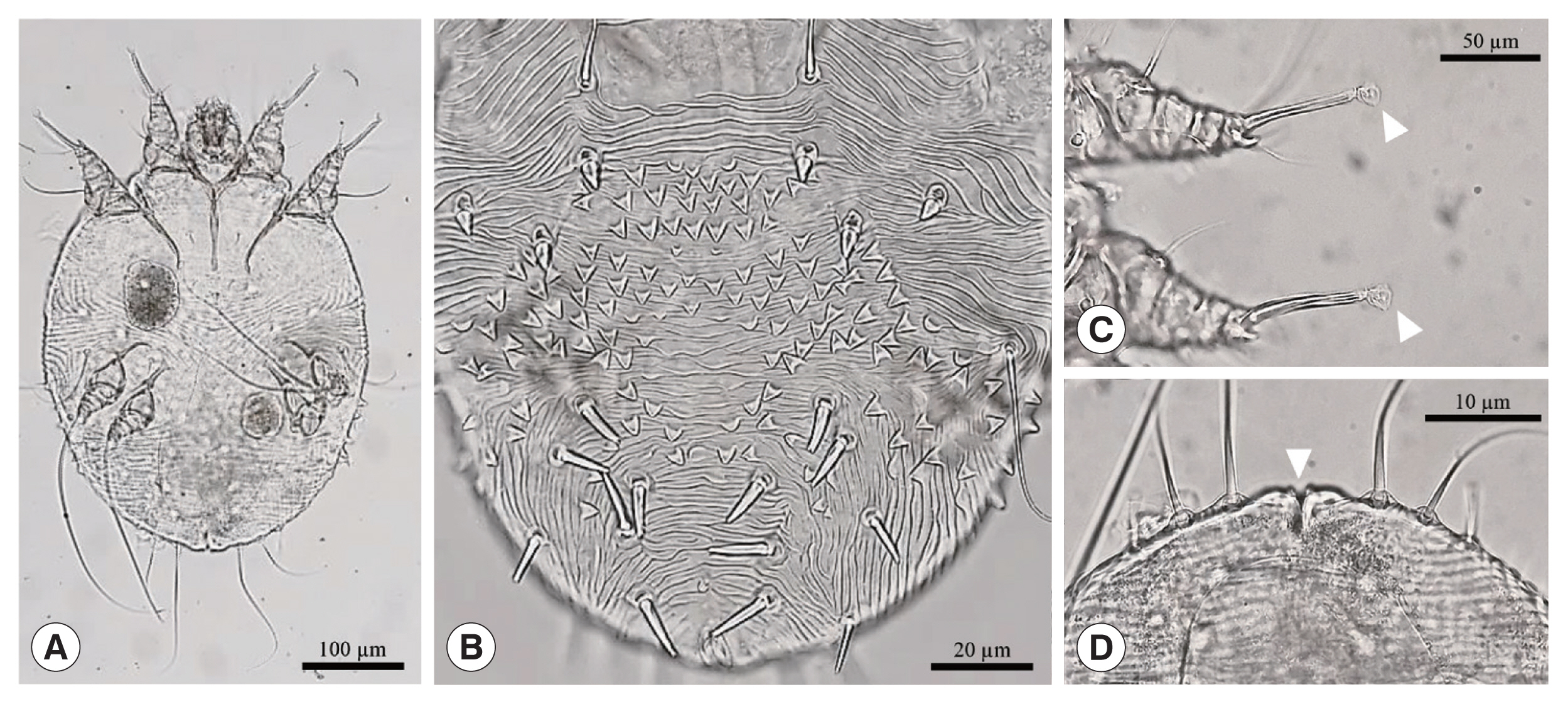
For microscopic examination, mites were incubated with 1 M KOH at 65°C for 45–60 min. Following incubation, 50–100 μl of the solution were plated on a microscope slide, covered with a cover slip, and examined under 1009 magnification in a light microscope (Olympus BX-50, Tokyo, Japan). Several ectoparasites recovered from 2 long-tailed gorals showed the general morphology of S. scabiei (Fig. 2). The overall shape was spherical, oval, and tortoise-like body. The ventral side of the mite was flat and dorsal side was convex. There were dorsal spines, coarse striations, and internal scapular lamellate setae. They had 4 pairs of legs (4 in front and 4 in rear) with several cuticular spines. There were several short bristles in the body and long ones in rear legs where legs had suckers [4].
Genomic DNA was extracted from individual mite using mini kit QIAamp DNA (Qiagen, Hilden, Germany) for the genetic identification and phylogenetic analysis of S. scabiei. PCR to amplify a 396 bp region of the COX1 (cytochrome c oxidase I) gene was performed using the forward primer 5′-GACACCCAGAAGTTTACATTC-3′ and the reverse primer 5′-TATATTTTGATAATGAATCTC-3′ in a mixture (50 μl) consisting of 50–100 ng genomic DNA, 1×PCR buffer, 0.4 mM each dNTP, 0.3 μM each primer, and 1 unit KOD FX Neo polymerase (Toyobo, Osaka, Japan). PCR amplification was performed in a T100 thermal cycler (Bio-Rad, Singapore) with an initial denaturation at 94°C for 3 min, 33 cycles of 98°C for 10 sec, 50°C for 30 sec, 72°C for 45 sec, and a final extension of 72°C for 5 min. The PCR product was analyzed by 1.5% agarose gel electrophoresis, sequenced (Bioneer, Seoul, Korea), and used for species identification using the NCBI BLAST program (https://blast.ncbi.nlm.nih.gov). Sequences were aligned using CLUSTAL [6]. The phylogenetic relationships were inferred using maximum likelihood analysis with MEGA software version X [7]. The evolutionary distance was calculated according to the algorithm of the Tamura-Nei model [8]. Bootstrap analysis was done with 1,000 replications.
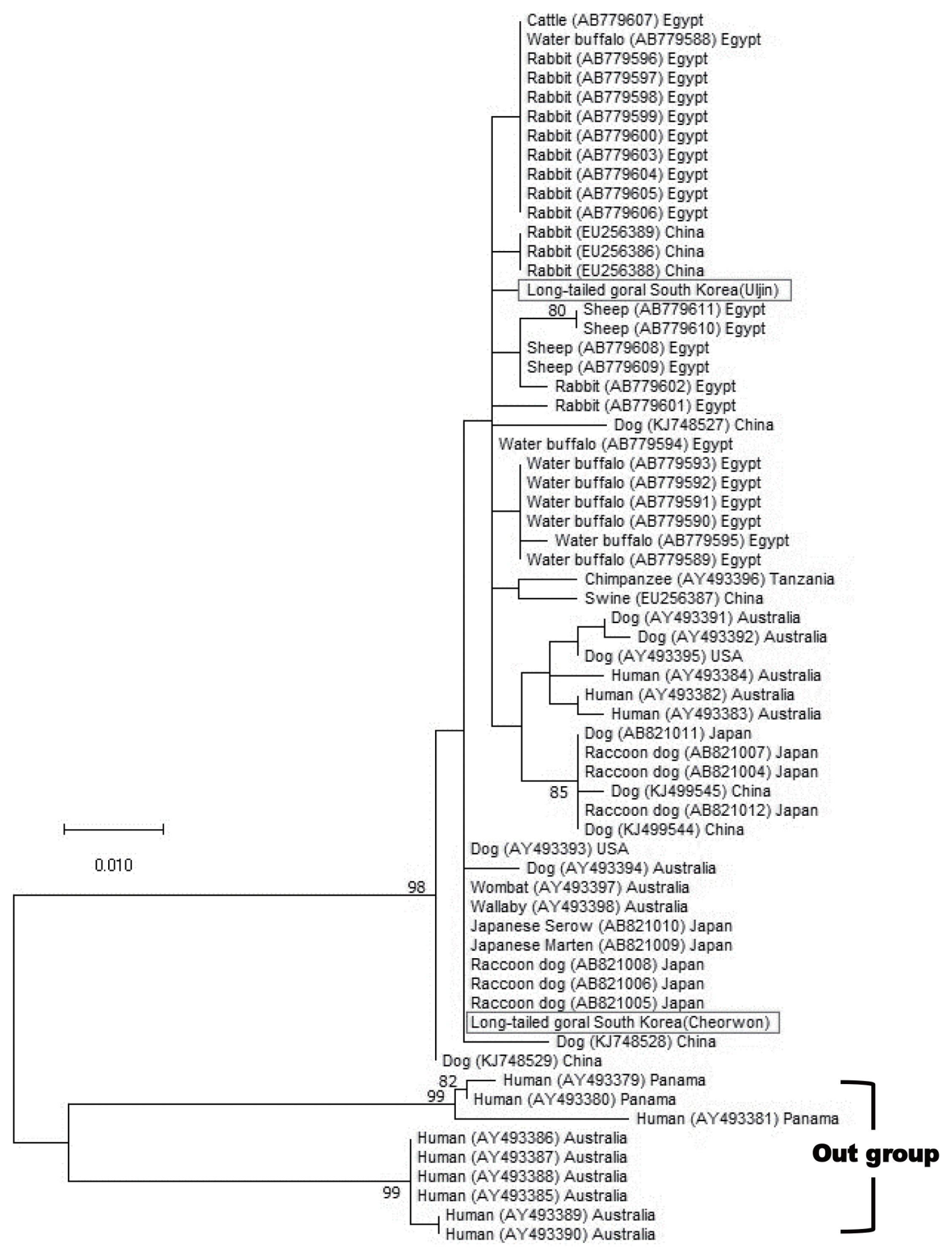
NCBI BLAST searches showed that the COX1 sequence of our mite was 99.6–100% identical to S. scabiei (MN349096). We identified this mite as S. scabiei. To determine whether S. scabiei isolated from long-tailed gorals had genetic variations, the COX1 gene was used as a genetic marker. Our 396 bp-long COX1 sequence was compared with the S. scabiei sequence identified in a previous study [9]. The phylogenetic tree of S. scabiei COX1 from the 2 rescued long-tailed gorals did not show a correlation between host preference and genetic variation. Furthermore, they exhibited some genetic distance in the S. scabiei’s phylogenetic tree using a maximum-likelihood model (Fig. 3).
DISCUSSION
The present study reported the first case of S. scabiei infestation in 2 long-tailed gorals from 2 different regions of Korea. We confirmed the species of the parasites using morphological and genetic tools. Scabies genes exhibited some variations between these 2 regions. It was a rare case in which there was insufficient empirical evidence for the endangered long-tailed mountain goat in Korea. Our study raises potential processes of interspecies infection (e.g., intraspecies or predator interactions) in relation to the spread of wildlife diseases (e.g., S. scabiei) and conservation of endangered ungulates in this country.
Recently, the frequency of observing long-tailed gorals in new regions has increased. It has been observed in northern Gyeonggi-do (Pocheon-si (si=city), Gapyeong-gun, Dongducheon-si, and Yeoncheon-gun), Seoul (Yongmasan and Inwangsan), Gangwon-do (Cheorwon-gun and Chuncheon-si), and Gyeongsangbuk-do (Juwangsan, and Pohang-si). Their distribution seems to have originally spread from Gangwon-do to the southwest regions. The expanding distribution pattern of long-tailed gorals might be related to their increasing population size. In Korea, the population size of long-tailed gorals was estimated to be more than 1,582 based on a recent monitoring data (2010–2020). There might be a total of 2,000 individuals if some non-surveyed areas (i.e., Demilitarized Zone and Civilian Control Zone) were considered. Since the expansion of the distribution of long-tailed gorals in this country often overlaps with other host species such as wild raccoon dogs (Nyctereutes procyonoides), wild boars (Sus scrofa), and feral dogs, the possibility of cross-infection with S. scabiei cannot be ruled out. For instance, wild raccoon dogs and free-ranging stray dogs have previously been diagnosed to have scabies in Korea [10–12].
Many genetic studies have suggested that the potential interaction between host species and host geographic location might shape the phylogeny of S. scabiei [13]. In Korea, a literature review mentioned that sarcoptic mange was assigned as one of the 6 diseases or disease agents that could influence on wildlife species [14]. Hypersensitivity reactions of wild raccoon dogs and feral dogs to S. scabiei was commonly reported in Korea. It is known that the numbers and spaces of these dwelling animals are also expanding [10,11]. In Japan, epizootics of sarcoptic mange in raccoon dogs might be related to the high density of the host population [15]. Furthermore, interspecific infectivity of S. scabiei can induce different hypersensitivity among species in sympatric prey-predator interactions (e.g., red deer, roe deer and chamois in ungulate preys versus predators, wolf and red fox) in Europe [16]. Considering that long-tailed gorals do not have major predators, the source of direct cross-infectivity of S. scabiei is uncertain. Future study should collect empirical evidences on habitat overlapping of long-tailed gorals with other wild animals such as wild boars, Korean water deer, raccoon dogs, and/or feral dogs in Korea.
Interactions between ungulates and sarcoptic mange might affect population dynamics of endangered species. A systematic plans for control and management of wildlife diseases is highly required. For example, Iberian ibex (Capra pyrenica) endemic to the Iberian Peninsula underwent overabundance and range expansion, followed by the outbreak of sarcoptic mange [17]. The sarcoptic mange could negatively influence the population dynamics of mountain ungulates (Caprinae) among other wild hosts, resulting in variable mortality (e.g., disease spread rate ca. 6 km per year, mortality rate >95.0%) [18,19]. It is noteworthy that S. scabiei with multi-host dependence is also likely to exhibit the spatiotemporal pattern of sarcoptic mange parasitic on long-tailed goats [20]. Disease control and management of long-tailed goats can utilize multifaceted biomonitoring tools including visual diagnosis, photo traps, trained dogs, body temperature measurements, immunodiagnostics, molecular tools and epidemiological modeling [18]. Health protocols (e.g., supplemental nutrition and vaccination) may be available during energy-demanding life cycle, food shortages, and/or heavy snowfall period [4,21]. With respect to hypersensitivity and cross-infectivity of sarcoptic mange in long-tailed gorals, uncertainty still remains due to a long-term bio-surveillance in the wildness. Individual mortality may be important as sarcoptic mange is unlikely to affect the long-term dynamics in population maintenance of long-tailed goats.
In conclusion, the present study confirmed that the mange mite was the pathogenic scabies in long-tailed gorals, but such a result has never been reported in other countries. Investigating the prevalence of mange mites in the wild population is difficult because it requires sampling from large numbers of long-tailed goral. Future studies should focus on whether sarcoptic mange has serious consequences in small, fragmented populations such as vulnerable long-tailed goral.