INTRODUCTION
Leishmania amazonensis, a member of the Leishmania mexicana complex, has been identified from patients with diverse clinical forms, including cutaneous leishmaniasis, diffuse cutaneous leishmaniasis (DCL), and visceral leishmaniasis in South American countries [1]. Patients with DCL are often resistant to chemotherapy and defective for the leishmanin skin test or antigen-stimulated T-cell proliferation in vitro, but they remain responsive to other antigens, such as tuberculin and lepromin [2]. The mechanisms responsible for this specific suppression of cell-mediated immune responses remain obscure, although it has been suggested that epidermal deficiencies of cytokines and cytokinemediated accessory signals may play a role in DCL. The regulation of host immune responses to Leishmania parasites has been well defined in cutaneous Leishmania major infections in inbred strains of mice that are either genetically susceptible (BALB/c mice) or resistant (most mouse strains) to the parasite. In this model, dominant Th1 type responses lead to the control of infection in resistant mice and early burst of IL-4 determines disease progression in susceptible hosts [3]. Expansion of a Th1 subset of CD4+ lymphocytes that secretes IFN-γ and IL-2 is associated with resistance to infection and IL-12, likely secreted by antigenpresenting cells (APCs), enhances expansion of Th1 cells through a pathway involving natural killer (NK) cells [4]. NK cells are a source of interferon that drives differentiation of CD4+ T cell subsets and induces early resistance of mice to L. major infection [5]. During the course of L. amazonenesis infection, BALB/c and C57BL/6 mice do not mount a vigorous Th2 response, but they do generate detectable levels of Th1-type responses [3]. However, Ji et al. [3] revealed that there is profound impairment in multiple immune functions at early stages of infection with L. amazonensis and adoptive transfer of Th1 cells would restore gene expression and control of parasite growth.
In this study, we tried to identify the effects of Aureobasidium-derived soluble branched (1,3-1,6) β-glucan (Sophy β-glucan) in L. amazonensis infection. Sophy β-glucan is a new type β-1,3-1,6 glucan synthesized from the black yeast, Aureobasidium pullulans, strain AFO-202. It is prepared by the Sophy Co. (Kochi, Japan) using the latest biological culture techniques (Ikewaki et al.; United States Patent 6956120 and Japan Patent 2004-329077) and is currently available in the market as a health food supplement. The β-glucans are originally extracted from the common baker's yeast, Saccharomyces cervisiae [6], some types of seaweed [7], and mushrooms such as Basidiomycetes spp. Reishi, Shiitake and Maitake [8,9]. They are potentreticuloendothelial-modulating agents whose immunobiological activity is mediated by stimulating proinflammatory cytokine production, in part, by an increase in the number and function of macrophages [10], modulation of immunity, and resistance to microbial challenge [11]. The β-glucans can greatly increase numbers of phagocytes for phagocytosis and induce the specific receptor expression to be presented onto the cell surface [12], such as Dectin-1(β GR) and Toll-like receptors (TLR-2) [12-14], which recognize and bind to foreign invaders, such as microbes, viruses, yeasts, parasites, and neoplastic cells. However, Dectin-1 is thought to be the main β-glucan receptor involved in innate and adaptive immune responses to foreign antigens and pathogens, including β-glucan as an immune function stimulator [15]. Ikewaki et al. [16] proved that Dectin-1 is the Sophy β-glucan receptor with the cooperation of TLR-2 and TLR-4. Their study also proved that Sophy β-glucan has immunoregulatory functions and act as a biological response modifier.
Administration of oat β-glucan by intragastric or subcutaneous routes to mice enhanced their resistance to Eimeria vermiformis infection, further demonstrating its immune stimulating activity [17]. Gaforio et al. [18] showed that Tilorone which is widely used to boost NK cell activity in mice increases the capacity of splenic macrophages to phagocytize C. albicans through activation of NK cells. The presence of NK cells is essential in order to maintain a basal level of phagocytic activity of splenic macrophages in control mice. Also in L. amazonensis infection, splenic macrophage activity should be activated to improve the phagocytic function by increased nitric oxide (NO) production to give a protection. Sophy β-glucan can be a good immunomodulator in L. amazonensis infection because it acts via Dectin-1 receptor to improve the macrophage activity and also boost NK cells that help to improve the capacity of splenic macrophages to phagocytize Leishmania parasites.
MATERIALS AND METHODS
Animals
Six-week-old female C57BL/6 and BALB/c mice were used. Animals were maintained at the animal house of Kochi Medical School in specific pathogen-free (SPF) conditions. This experiment was performed under institutional animal guidelines. Control groups were supplied with water, and study groups were supplied with 5% Sophy β-glucan (prepared in the laboratory of Sophy Co.) as an oral supplement freely in SPF conditions throughout the study. They were fed with conventional feed ad libitum.
A basal experiment was performed without infection of L. amazonensis to check whether Sophy β-glucan has an effect on NK activity in C57BL/6 mice because this mice strain is considered to be having high NK activity and Th1 response compared to BALB/c mice. The results of this experiment clearly indicated that the NK activity increased over time in the Sophy β-glucan supplemented group, whereas it returned to normal level after 1 week of the withdrawal of the supplement.
L. amazonensis infection
L. amazonensis (MHOM/BR/73M2269) was cultured in RPMI medium with 10% fetal calf serum for 1 week at 25℃. Stationary-phase promastigotes (1 × 107 cells in PBS) were injected into the left footpad of the study group mice and PBS was given to the control mice. The infected mice were divided into 2 groups, and 1 group was supplied with water and the other group was given Sophy β-glucan continuously. The footpad thickness of these mice were measured with a digital micrometer at each week postinfection (PI) up to 8 weeks and the final batch at week 12 PI, and sacrificed at each point to measure the NK activity. BALB/c mice were used only up to week 4 PI and C57BL/6 mice were continued up to week 12 PI.
Assay for NK cytotoxicity
Spleen cells of control and infected mice were harvested each week PI until week 8 and final batch at week 12 PI and were checked for NK activity. NK activity of effector cells were measured by a 4-hr 51Cr-release assay using labeled YAC-1 target cells. Radioactivity was measured by Packard Biogamma Counting system (LaGrange Illinois, USA). Percentage of cytotoxicity, as measured by specific 51Cr-release, was calculated using the formula; 100 × (experimental cpm - spontanous cpm)/(maximal cpm-spontaneous cpm).
Cytokine production
Serum samples of control and infected mice were checked for IL-4 and IFN-γ production using sandwitch ELISA. ELISA kits (Bioscience, Meriden, Connecticut, USA) were used according to the manufacturer's instructions.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was carried out using the non-parametric ANOVA test and SPSS program version 13.0 (SPSS Inc., Illinois, USA). The data are representative of 3 experiments. The differences were examined by using the Student's t-test as well. Differences were considered significant if P-value was less than 0.05.
Results
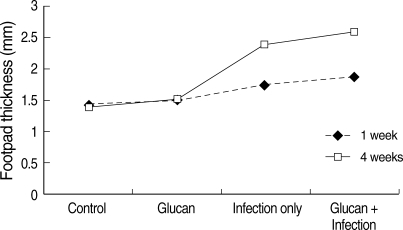
In the L. amazonensis infection study, increase in the footpad thickness was observed in BALB/c mice with time of infection (Fig. 1). There was a significant difference in mean footpad thickness in BALB/c mice among the 4 groups of experimental mice based on univariate analysis of variance using a generalized linear model (R2 = 0.64; P < 0.001). A significant difference between the footpad thickness at week 1 and 4 PI was also observed (P < 0.05). At week 1 PI, the footpad thickness was maximum in mice that belonged to the 'infection + glucan' group (mean ± SD = 1.94 ± 0.12 mm) compared to the 'infection only' (1.76 ± 0.07 mm) and control (1.43 ± 0.04 mm) groups. There was no difference in the footpad thickness in control and 'glucan' only groups. There was an increase in the foot pad thickness in 'infection + glucan' treated mice in week 4 PI (2.58 ± 0.15 mm) compared to the foot pad thickness in 'infection + glucan' group at week 1 PI (1.94 ± 0.12 mm). The mean foot pad thickness in 'infection only' group at week 4 PI was lower (2.43 ± 0.32 mm) compared to 'infection + glucan' group (2.58 ± 0.15 mm) and control group of mice (1.46 ± 0.05 mm). The temporal variations in mean footpad thickness in 4 groups of experimental C57BL/6 mice with time of infection are given in Fig. 2. The difference in overall mean foot pad thickness between 'infection only' group versus 'infection + glucan' group of mice was statistically significant (Bonferroni post-hoc comparison; P < 0.001).
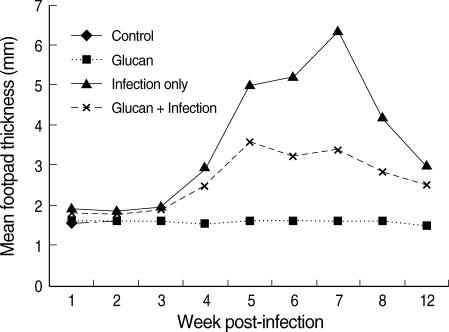
There were no changes in the foot pad thickness at different time points during the course of the study in 'control' and 'glucan only' group of mice. However, maximum footpad thickness was observed in mice which belonged to the group 'infection only'. The mean footpad thickness in C57BL/6 mice at week 1 PI was the highest in the 'infection only' group (1.9 ± 0.15 mm), whereas in infected mice treated with glucan, the mean footpad thickness was lower (1.76 ± 0.15 mm). There was a remarkable increase in the footpad thickness from week 3 PI with a peak footpad thickness observed in week 7 PI in 'infection only' group (6.3 ± 0.91 mm) compared to the 'infection + glucan' group (3.3 ± 0.16 mm). On the other hand, the footpad thickness at different points of time was markedly less (based on at least 3 replicates at each point of time) in mice which belonged to the group 'infection + glucan' compared to the 'infection only' group of mice (Fig. 2). The footpad thickness started to decrease after week 7 PI in both groups until it reached to mean footpad thickness (2.54 ± 0.20 mm) at week 12 PI in 'infection + glucan' group compared to 'infection only' (3.0 ± 0.40 mm) mice. Difference in the footpad thickness was observed between these groups at this point of time although it was not statistically significant.
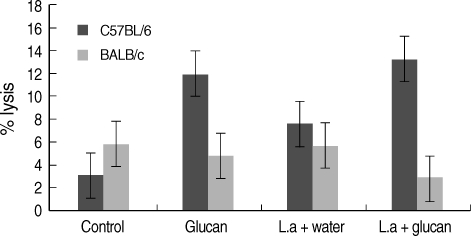
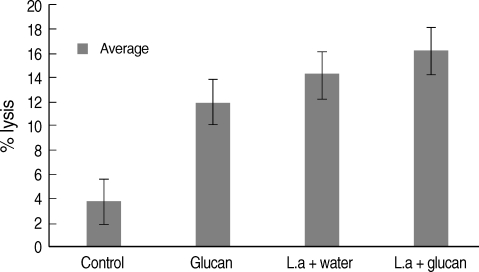
NK activity was also measu1red in each week after infection (figures given for week 1, 4, 8, and 12 PI) and a higher activity was revealed in C57BL/6 mice compared to BALB/c mice group. In BALB/c mice, the mean NK activity at week 1 PI was found to be the highest in the 'control' group of mice (mean ± SD = 3.0 ± 0.87) compared to 'glucan only' group (1.43 ± 1.43) (Fig. 3). NK activity in mice belonging to 'infection only' group was high (1.64 ± 0.45) compared to 'infection + glucan' group (1.17 ± 0.52). In C57BL/6 mice NK activity at week 1 PI was high in 'glucan only' group (5.59 ± 0.53) compared to 'control' group (3.88 ± 0.34) followed by the highest activity seen in 'infection + glucan' group (6.63 ± 0.96) compared to 'infection only' group (6.0 ± 0.06), but the differences were not statistically significant. There was a marked decrease in NK activity in 'infection + glucan' treated group of BALB/c mice (2.81 ± 0.60) at week 4 PI compared to 'infection only' group (5.67 ± 0.93), and higher activity was observed in 'control' group (5.88 ± 1.4) compared to 'glucan only' group (4.88 ± 0.7) (Fig. 3). In C57BL/6 mice, NK activity at week 4 PI was markedly increased in 'infection + glucan' group (13.3 ± 3.1) than week 1 PI. There was a significant difference in NK activity between 'control' (3.5 ± 0.49) and 'glucan only' (11.8 ± 0.63) groups and also between 'infection only' (7.56 ± 2.6) and 'infection + glucan' groups (13.3 ± 3.1) (P < 0.05) (Fig. 3). According to the 3 replicates of this experiment, NK activity in C57BL/6 mice increased with time. At week 8 PI, NK activity was high in 'infection + glucan' group compared to 'infection only' group, but the difference was not significant (Fig. 4). Mean NK activity in 'glucan only' group was high compared to 'control' group at this time showing a significant difference (P < 0.05). The highest NK activity was seen at week 12 PI in 'infection + glucan' group (Fig. 4) and the mean NK activity was high (16.2 ± 3.1) compared to 'infection only' (13.675 ± 1.51) group, but the difference was not statistically significant. Mean NK activity in 'glucan only' group was high (14.0 ± 5.81) at this time compared to 'control' group (3.75 ± 0.95) showing a significant difference (P < 0.05). However, difference in the NK activity between 'control' and the 'infection + glucan' group was also statistically significant at this time (P < 0.05).
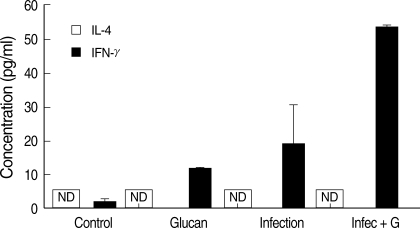
IFN-γ (a Th1-cytokine) was not detected in BALB/c mice sera during the study period and it also did not increase to a significant level in C57BL/6 mice up to week 7 PI. The highest level of IFN-γ was observed in the sera of 'infection + glucan' group of C57BL/6 mice at week 7 PI, and it was significantly higher than the 'infection only' group (P < 0.05) (Fig. 5). There was also a difference in the IFN-γ levels between 'control' and 'glucan only' groups during this period. IFN-γ level started to decrease again at week 8 PI in the infected glucan treated group, but still was significantly higher than the infection only group (P < 0.05) (Fig. 5). At week 12 PI, IFN-γ level in the 'infection+ glucan' group was significantly lower than the 'infection only' group (Fig. 5). IL-4 levels in the sera of BALB/c and C57BL/6 mice were also measured, but no detectable levels were found throughout the study period (Fig. 5).
Discussion
Parenteral administration of botanical and yeast extracts containing (1,3)(1,6)-β-D-glucan has shown antimicrobial effects in animal studies and in patients with high risks of development of infections [19-21]. Although (1,3)(1,6) β-D-glucan was the primary focus of most previous investigations, it's in vivo immunomodulatory effects, especially when it is administered orally, have not been tested. Furthermore, β-glucan, despite its wide distribution in daily diets, has not been rigorously tested in purified forms. In the present study, we demonstrated that oral administration of (1,3-1,6) β-glucan (Sophy β-glucan), a soluble branched β-glucan derived from Aureobasidium species which contains about 10-fold the quantity of β-glucan than mushrooms extracts, protecting mice from infection of L. amazonensis by enhanced NK activity and cellular immunity.
Results of the basal experiment revealed that Sophy-β-glucan has time-dependent and dose-dependent effects on NK activity in C57BL/6 mice. NK activity was compared in C57BL/6 and BALB/c mice because the NK activity and Th1 responses are considered higher in C57BL/6 than BALB/c mice. Results of our experiment revealed that the NK activity was higher in glucantreated group of C57BL/6 mice that the control group and also BALB/c mice. With L. amazonensis infection, NK activity increased with time in C57BL/6 mice treated with Sophy β-glucan compared to BALB/c mice. High NK activity in these mice has resulted in more marked diminution in footpad swelling after 3 weeks of infection in 'infection + glucan' group compared to the 'infection only' group, and this may be due to an increase in elimination of parasites by improved splenic phagocytic activity due to β-glucan effects. The highest NK activity was observed at week 12 PI in 'infection + glucan' group of C57BL/6 mice and the footpad thickness also decreased to a mean level of 2.6 mm at this time. Decrease in the footpad thickness in 'infection only' group also after 7 weeks of infection may be due to activation of inflammatory cytokines by the infection to reduce the parasitic load as described by Ji et al. [3].
In BALB/c mice, increase in the footpad swelling with time of infection even in the 'infection + glucan' group might be due to a low NK activity and a low Th1 response, and also to the fact that they have not been activated by Sophy β-glucan supplement. There was no difference in the footpad thickness in control and the 'glucan only' groups of BALB/c mice, and the highest NK activity was also found in the control group. According to Figs. 1 and 2, there was a difference in the footpad swelling between BALB/c and C57BL/6 mice in spite of the supplement with Sophy β-glucan. We suggest that this might be due to an unknown mechanism present in these mice strains. Although the IFN-γ level was checked at each week of infection, the fact that IFN-γ level was not detected to a significant level until week 7 PI in C57BL/6 mice may be due to a delayed and depressed expression of inflammatory cytokines by L. amazonensis infection at early stage, as described by Ji et al. [3]. Also, the secretion of IFN-γ had not been activated by Sophy β-glucan supplement. High levels of IFN-γ at week 7 PI in the 'infection + glucan' group correlated with the highest foot pad thickness at this time indicating the maximumlevel of infection. Djeu et al. [22] also proved that antigens like viruses, bacterial products, and tumor cells are able to induce interferon (IFN) from NK cells and IFN-mediated boosting of NK activity. This may be the cause for increase of NK activity in 'infection only' group as well with time of infection because parasitic antigens can induce the release of IFNs to boost the NK cell activity. From week 8 PI, the IFN-γ level of 'infection + glucan' group started to decrease, and it was lower than the 'infection only' group at week 12 PI due to elimination of parasites by higher NK activity which enhanced the splenic-phagocytic function. High levels of IFN-γ in 'infection only' group at week 12 PI may be due to the presence of high parasitic load to activate inflammatory cytokines than in the 'infection + glucan' group. These results suggest that IFN-γ is secreted by NK cells at weeks 7-8 PI and by Th1 cells at week 12 PI, as described by Scharton et al. [5]. Their study indicated that the NK cells control early parasite numbers and facilitate early Th1 development in L. major infection, and the results of our study suggest a similar control in L. amazonensis infection. Therefore, NK activity is very important in the control of Leishmania infection.
Enhanced levels of NK activity with Sophy β-glucan treatment in C57BL/6 mice compared to BALB/c mice suggest that Sophy β-glucan boosts up NK activity by increasing the frequency of NK cells and also activates Th1 responses in C57BL/6 mice. Low levels of IL-4 in the infected mice sera support this suggestion. It can be concluded from our study that the Sophy β-glucan activates NK activity in L. amazonensis infection in C57BL/6 mice and gives a protection by activating Th1 response and the splenic macrophages' phagocytic function. This protection might have been enhanced further by Sophy β-glucan in L. amazonensis-infected mice as it has been proved that the Sophy β-glucan acts as a body response modifier as well. Further research is being carried out to check the Sophy β-glucan activity on other cytokines, such as IL-8, IL-10, IL-12, and TNF-a, and on IgG levels according to the time of L. amazonensis infection and also to check whether depletion of NK cells by administrating anti-asialo GM1 will reverse the protective effects of Sophy β-glucan. It is necessary to perform further studies for better understanding of Sophy β-glucan activity in order to make use of it in the treatment of leishmaniasis.